Skeletal myosin II is a motor protein essential for muscle contraction, converting chemical energy from ATP into mechanical force to slide actin filaments. It plays a central role in generating the tension required for voluntary movement, making it critical for locomotion and posture maintenance.
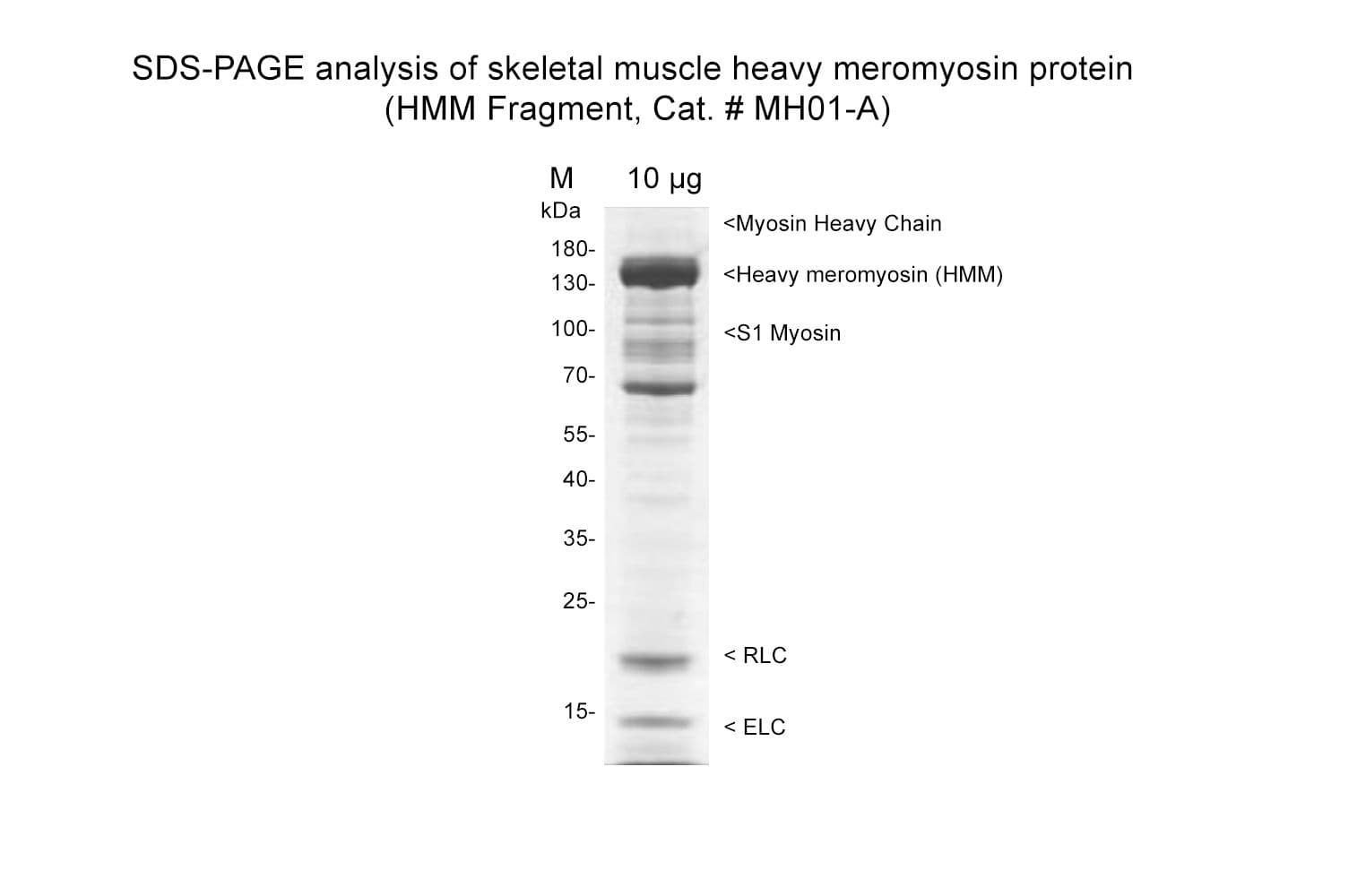
Skeletal muscle Heavy Meromyosin (HMM fragment) is prepared by digesting myosin II MY02 with -chymotrypsin in the presence of MgCl2 to liberate the soluble HMM. A final polishing step using anionic exchange chromatography is performed.
Protein purity is assessed by scanning densitometry of Coomassie Blue stained protein on a 4-20% polyacrylamide gel. Purity is determined to be >70% pure HMM
The biological activity of the bovine cardiac myosin HMM fragment is measured by its rate of F-actin–activated ATP hydrolysis. In the presence of F-actin, the ATP hydrolysis rate for MH01 is in the range of 500-600 nmol ATP hydrolyzed per minute per mg of HMM.
Cat. #MH01-A