Human KIF20A is a mitotic kinesin motor protein essential for proper chromosome segregation and cytokinesis, functioning by interacting with microtubules to drive spindle organization. It plays a key role in cell cycle progression, and its dysregulation has been linked to cancer proliferation and poor prognosis.
The wild-type human motor domain of KIF20A (MKLP2) is produced in a bacterial expression system.
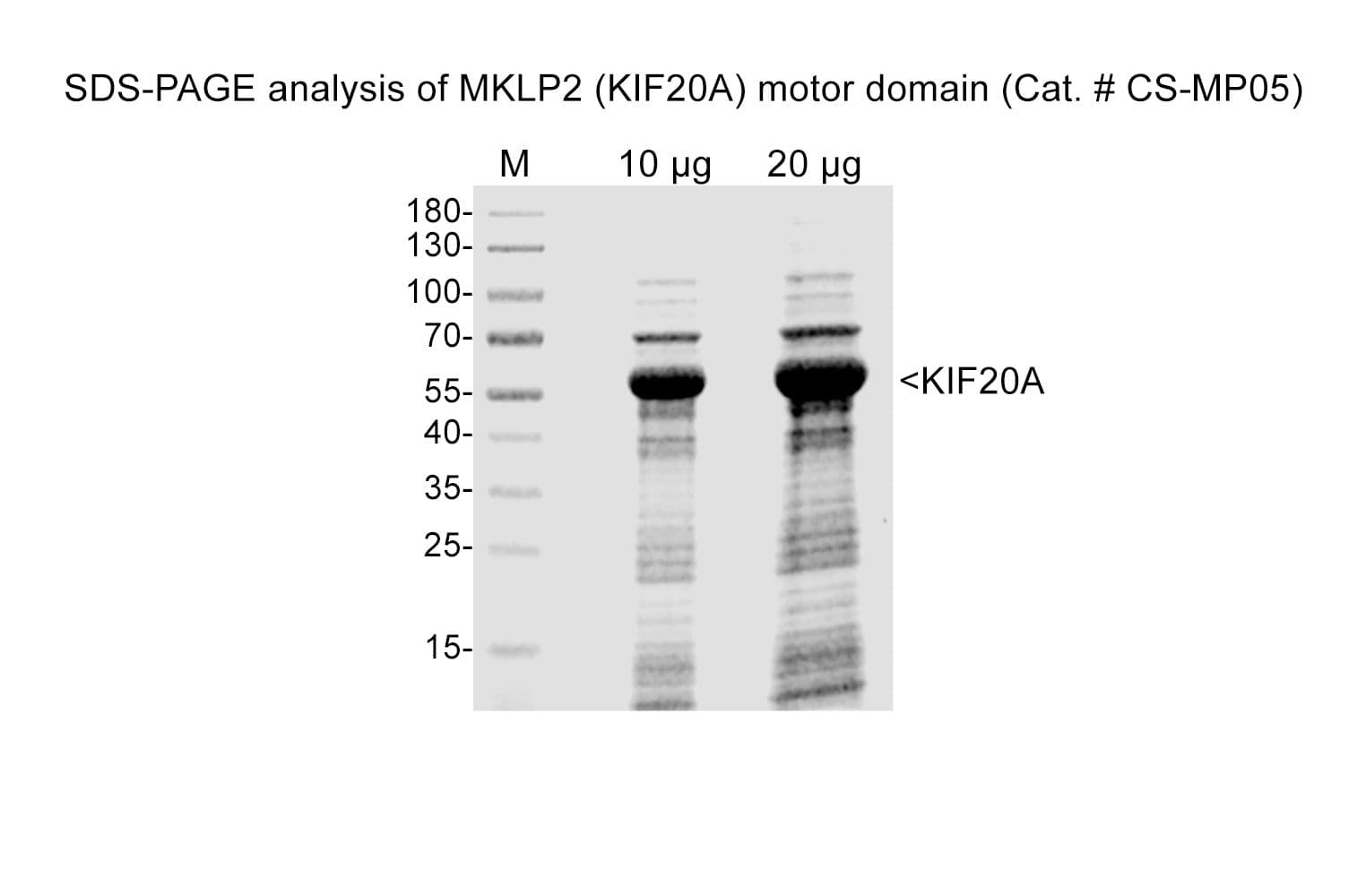
Protein purity is assessed by scanning densitometry of Coomassie Blue-stained protein on a4-20% polyacrylamide gel. Purity is determined to be >70% pure.
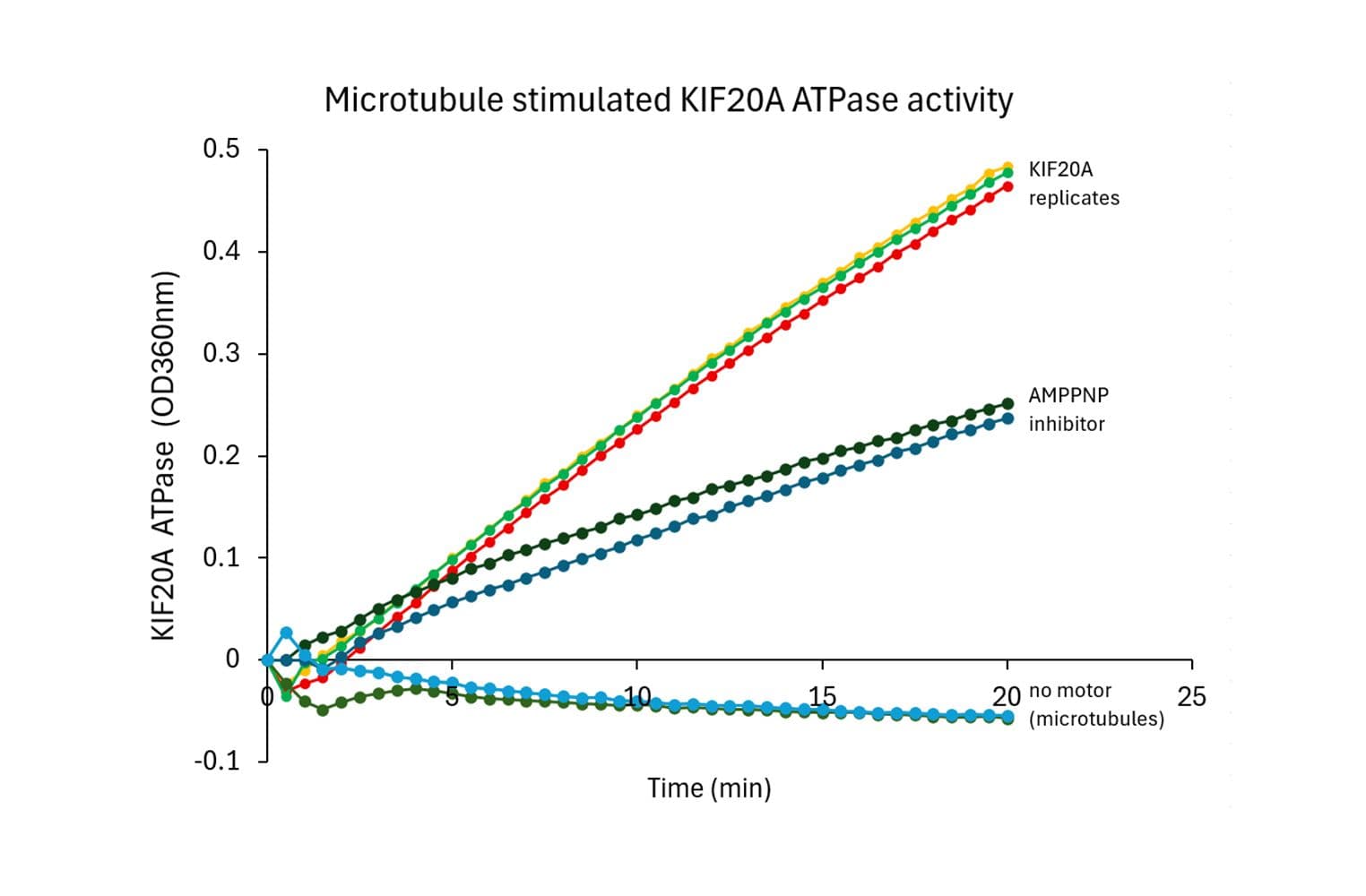
Biological activity of CS-KF20A is measured using a microtubule-activated ATPase assay. Under the conditions stated (see datasheet), KIF20A exhibits a microtubule-stimulated ATPase activity with a Vmax of ≥ 1330 nmol of ATP produced per minute per mg of KIF20A.
Cat. #CS-MP05