Abstract
We describe the results of a comparison between a newly developed phosphotyrosine specific antibody, clone 27B10.4 (Catalog # APY03) with the popular phosphotyrosine antibody clone 4G10. Antibody performance was compared in immunoprecipitation, immunofluorescence, and western blot applications. In all cases, the data shown are representative of at least 4 separate experiments. We report that 27B10.4 and 4G10 have a broad similarity in phosphotyrosine-protein recognition, with both antibodies showing some sequence context differences. Clone 27B10.4 was shown to immunoprecipitate a wider diversity of phosphotyrosine-proteins than 4G10 and to detect stronger signals in low molecular weight proteins in western blot applications. This, together with a superior performance in immunofluorescence applications, makes clone 27B10.4 an exciting new reagent for the study of phosphotyrosine regulation in health and disease.

Introduction
The post-translational modification (PTM) of tyrosine residues by the reversible addition of a phosphate group is a powerful signaling switch in a wide range of cellular events (1). Many growth factors act through receptor tyrosine kinases and subsequent phospho-tyrosine/serine/threonine cascades to control cell proliferation, migration, and adhesion (2). The deregulation of tyrosine phosphorylation is known to underlie many diseases including cancers and many of the 90 human tyrosine kinases are targets for the development of anti-cancer therapeutics (3). Some well known tyrosine kinase inhibitors include GleevecTM approved for the treatment of chronic myeloid leukemia (CML) and IressaTM and TarcevaTM for the treatment of non small cell lung cancer (NSCLC) (3).

Because of the critical importance of tyrosine phosphorylation in normal and aberrant cell functions, there is great interest in identifying the phosphotyrosine profile of single proteins, protein pathways and whole cells under a variety of conditions and dynamic states. Phosphotyrosine antibodies are a powerful tool in helping elucidate the role of this PTM in cellular functions.
Results
Immunoprecipitation Comparison
Clones 27B10.4 and 4G10 were used to immunoprecipitate phosphotyrosine-proteins in pervanadate-treated and untreated NIH3T3 cell lysates. Pervanadate is an irreversible inhibitor of the protein tyrosine phosphatase PTP1B (4). As phosphotyrosine proteins are not prevalent in untreated cells, pervanadate treatment results in a dramatic increase in phosphotyrosine-protein species. Pervanadate is made by activating sodium orthovanadate and should be used immediately (see Materials and Methods).
Protein G-bound anti-phosphotyrosine antibodies were used to enrich phosphotyrosine-proteins from 200 µg of cell lysate. Protein G-beads alone or bound to non-specific mouse IgG do not show any protein signal in an IP (data not shown). Clone 27B10.4 enriched a more diverse set of phosphotyrosine-proteins than 4G10.
Notably, high molecular weight proteins >200 kDa and phosphotyrosine-proteins in the range of 15-60 kDa are preferentially enriched using 27B10.4 (Fig. 1).
Figure 1: Immunoprecipitation with 27B10.4 and 4G10
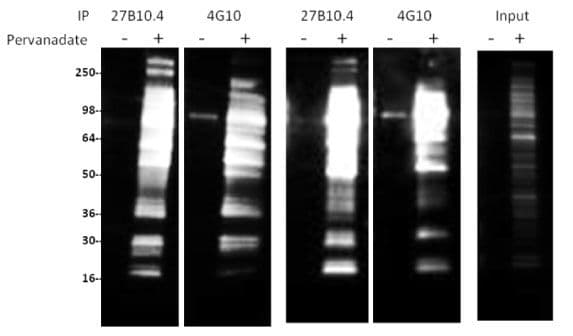
Legend: NIH3T3 cells were either treated (+) or untreated (-) with pervanadate (100 µM for 10min). 200 µg of lysate per reaction was used for immunoprecipitation of tyrosine-phosphorylated proteins. 27B10.4 or 4G10 (5 µl at 1 µg/µl) was first bound to protein G beads and then incubated with cell lysate. Western blots of immunoprecipitated proteins were developed using 27B10.4 at 1:500 dilution and CleanBlot (Thermo Scientific, #21230) at 1:1000 dilution) as secondary antibody. Input represents the signal from 5% of pervanadate treated or untreated NIH3T3 lysate.
Immunofluorescence Comparison
Clones 27B10.4 and 4G10 were used to detect phosphotyrosine-proteins in pervanadate-treated NIH3T3 cells. Both antibodies detected enhanced accumulation of phosphotyrosine-proteins in treated cells. Clone 27B10.4 consistently showed higher signal intensity than 4G10 (Fig. 2).
Figure 2: Immunofluorescence with 27B10.4 and 4G10
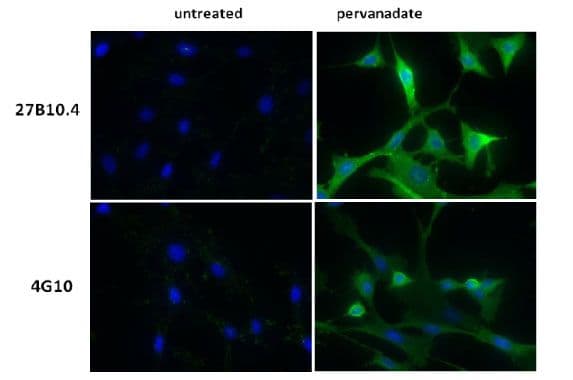
Legend: NIH3T3, untreated or treated with pervanadate (100 µM for 10 min), were stained with a 1:1000 dilution of phosphotyrosine antibody as described in Material and Methods. Phosphotyrosine and nuclei were visualized in green fluorescence and blue DAPI staining, respectively. Top images are from cells probed with 27B10.4, bottom set of images are from cells probed with 4G10.
Western Blot Comparison
Clones 27B10.4 and 4G10 were used to detect phosphotyrosine- proteins in lysates made from pervanadate-treated or untreated NIH3T3 cells. Specific enhancement of phosphotyrosine-proteins is detected in the treated cell lysates demonstrating that both antibodies showed strong specificity for phosphotyrosine-proteins (Fig. 3; compare + and - lanes). Banding patterns showed great similarity between the two antibodies (Fig. 3), demonstrating a high degree of overlap in antibody specificity. Interestingly, 4G10 Ab was more sensitive than 27B10.4 at detecting proteins in the >250 kDa range, while 27B10.4 gave a stronger signal for a set of proteins of low molecular weight around 16 kDa. This observation was seen in at least 4 repetitions of the western blot application.
Figure 3: Western Blot Analysis with 27B10.4 and 4G10
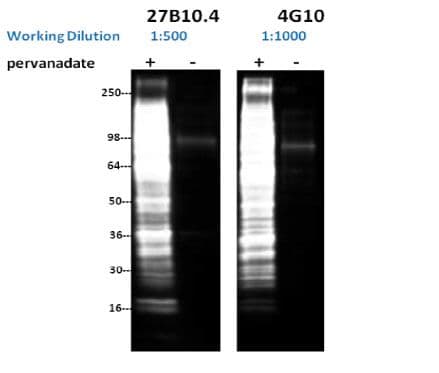
Legend: NIH3T3 cells were either treated or untreated with pervanadate (100 µM for 10 min.). 16 µg of each lysate was resolved in SDS-PAGE and proteins were transferred to PVDF membrane. Membranes were probed with a 1:500 dilution (2µg/ml) of 27B10.4 or a 1:1000 dilution of 4G10 (1 µg/ml).
Discussion
Despite its huge physiological importance, phosphotyrosine PTMs account for around 0.05% of the total phospho-protein population (5). This makes the more general phospho-enrichment techniques, such as immobilized-metal affinity chromatography (IMAC), employed in proteolytic/mass spectroscopy proteomics less useful for phosphotyrosine-proteins or peptides as they are likely to be obscured by the much more prevalent phosphoserine/threonine species (6). Hence, a phosphotyrosine antibody reagent is often employed as an enrichment step prior to mass spectroscopy analysis (6). Our data indicate that clone 27B10.4 will be a highly useful reagent in this application.
Great strides have been made in the development of phospho-specific antibodies, however, the use of a pan-phosphotyrosine antibody, coupled with immunoprecipitation of the protein under investigation, is still often the only way to track the phosphorylation of many proteins, particularly if multiple phosphorylation sites are involved. In this study, clone 27B10.4 was demonstrated to enrich a more diverse set of phosphotyrosine-proteins than 4G10, making it a highly desirable reagent in this application.
Clone 27B10.4 was demonstrated to perform comparably to clone 4G10 in western blot applications, however it appeared that, while the protein profile showed a high degree of overlap between the clones, there were clear differences in the antibody specificity (Fig. 3). The finding that phosphotyrosine antibodies show some sequence context sensitivity has been reported previously for phosphotyrosine antibodies, including clone 4G10 (7) and appears to also apply to clone 27B10.4. It is anticipated that clone 27B10.4 will be a valuable and novel addition to the arsenal of reagents available for western blot applications.
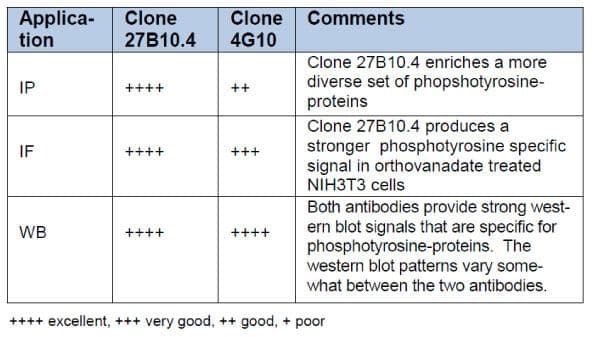
In conclusion, we have developed a new anti-phosphotyrosine mouse monoclonal antibody, clone 27B10.4 that specifically recognizes proteins post-translationally modified by phosphorylation of tyrosine residues. 27B10.4 was raised against a proprietary mixture of phosphotyrosine peptides conjugated to KLH. Comparison of 27B10.4 with the popular clone 4G10 has demonstrated that 27B10.4 has several advantages over 4G10 and is a useful new reagent for the investigation of tyrosine phosphorylation in a wide range of applications (Table 1).
Table 1: Summary of Antibody Comparisons
Materials and Methods
Reagents
Clone 27B10.4 was developed and manufactured at Cytoskeleton Inc. and is sold under Cat.# APY03, Clone 4G10 was purchased from Millipore (Cat. # 05-321). CleanBlot, Cat# 21230, and chemiluminescence detection reagent SuperSignal West Dura Extended Duration Substrate, Cat# 34076, was purchased from Thermo Scientific. Alexa-Fluor 555 goat anti-mouse antibody was from Life Technologies, Cat # A-21424. HRP conjugated goat anti-mouse IgG was from Jackson Immunoresearch Labs, Cat# 115-035-068. Protein G-beads were from BioVision, Cat# 6511-100. NIH3T3 cells were purchased from ATCC, Cat# CRL-1658. Sodium orthovanadate, Cat# 450243, and all other reagents were purchased from Sigma.
Immunoprecipitations
For immunoprecipitations, 5 µg of antibody was added to 500 µl of PBS containing 30 µl of protein G-beads. This was gently rotated for 1h at 4°C and then briefly washed three times in PBST (500 µl per wash). The antibody bound beads were pelleted by centrifugation (960 x g, 4°C, 1 min) and 200-500 µg of cell lysate (0.5 mg/ml protein in RIPA buffer: 50 mM Tris pH7.5, 1% IGEPAL, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate) was added to the beads. The reaction was gently rotated at 4°C for 1h. The beads were then pelleted at 960 x g, 4°C for 1 min. and washed three times in 50 mM Tris pH7.5, 150 mM NaCl, 1% IGEPAL (800 µl per wash). Phosphotyrosine-proteins were released from the beads by resuspension in 30 µl of 2X non-reducing SDS sample buffer (125mM Tris pH6.8, 20% glycerol, 4% SDS, 0.005% Bromophenol blue) and incubated at room temperature for 5 min. The beads were pelleted at 960 x g, 1 min, RT. The supernatant containing phosphotyrosine-proteins was removed to a fresh microfuge tube and 1 µl of beta mercaptoethanol was added to reduce protein disulfide bonds. Samples were boiled for 5 min and loaded onto SDS-PAGE for subsequent western blot analysis. To avoid detection of antibody heavy and light chains, CleanBlot was used as the secondary antibody detection reagent.
IF method
NIH3T3 cells were grown on acid-washed coverslips to a confluency of 30-40%. Cells were untreated or treated with pervanadate (100 µM for 10 min).Cells were fixed by dipping coverslips in –20°C methanol for 5 min. Coverslips were then dipped into –20°C acetone for 2 min. and air dried for 10 min. Cells were rehydrated by dipping the coverslips in PBS for 2 min. before addition of 100 µl of primary antibody (1:1000 dilution in PBS) and incubation at RT for 1h. Coverslips were washed three times (1 min each wash) in PBS (200 µl per wash). Secondary antibody at 1:500 dilution (Alexa Fluor 555) plus DAPI at 100ng/ml were placed on the cells and incubated at RT for 1h. Coverslips were washed with PBS as described above, rinsed once in sterile water (200 µl), and then dipped into RT absolute ethanol for 2 min before being allowed to dry. Coverslips were placed face down on glass slides with mounting media containing antifade and cells were observed by fluorescence microscopy.
Western blot method
Samples were run on SDS-PAGE using 4-20% Tris-Glycine gels. Gels were equilibrated in western blot buffer (25 mM Tris pH8.8, 192 mM glycine, 5% methanol) for 15 min at RT prior to electroblotting. The proteins were transferred to a PVDF membrane overnight at 40V and 4°C. Membranes were washed once in TBST for 10 min and membranes were blocked in 5% milk in TBST for 60 min at RT with constant agitation. The membrane was then incubated with a 1:500 dilution (27B10.4) or a 1:1000 dilution (4G10, manufacturers recommended dilution) of antibody in TBST at RT for 1h with constant agitation. Membranes were washed three times in TBST for 10 min each at RT, then incubated with a 1:20,000 dilution of HRP-conjugated goat anti-mouse IgG in TBST/3% milk for 30 min at RT with constant agitation. Membranes were washed 6 times in TBST for 10 min each and HRP signal was detected by chemilluminescence.
Activation of Sodium Orthovanadate to Pervanadate
Sodium pervanadate should be made fresh and used immediately. Pervanadate is made by adding 6 µl of hydrogen peroxide to 44 µl of PBS in a microfuge tube. Add 50 µl of 200 mM sodium orthovanadate (the solution will turn yellow) and use immediately at the recommended dilutions.
References
- Lim W. and Pawson T. 2010. Phosphotyrosine signaling: evolving a new cellular communication system. Cell 142:661-667.
- Wagner MJ et al. 2013. Molecular mechanisms of SH2– and PTB– domain-containing proteins in receptor tyrosine kinase signaling. Cold Spring Harb. Perspect. Biol. 5:a008987.
- Hunter T. 2009. Tyrosine phosphorylation: thirty years and counting. Curr. Opin. Cell Biol. 21:140-146.
- Huyer G. et al. 1997. Mechanism of inhibition of protein tyrosine phosphatases by vanadate and pervanadate. J. Biol. Chem. 272:843-851.
- Hunter T and Sefton BM. 1980. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc. Natl. Acad. Sci. USA 77:1311-1315.
- Johnson H and White F. 2012. Towards quantitative phosphotyrosine profiling In Vivo. Semin. Cell Dev. Biol. 23:854-862.
- Tinti M et al. 2012. The 4G10, pY20 and p-TYR-100 antibody specificity: profiling by peptide microarrays. New Biotech. 29:571-577.