Anti-Phosphotyrosine Validated Methods


Tyrosine phosphorylation, a reversible process, is one of the most frequent post-translational modifications of proteins and is crucial in mediating signal transduction in eukaryotic cells after exposure to cytokines and growth factors (1). Anti-phosphotyrosine antibodies have been an important tools in studying the level of tyrosine phosphorylation of proteins in different cellular models. It has also played an important role in enriching phosphotyrosine peptides from trypsin-digested cell lysates. As a result, a large number of phosphopeptides have been identified under various physiological and pathological conditions with mass spectrometry technologies (2-3).
The following protocols provide optimized techniques for detecting proteins with a phosphotyrosine residue in Western blot, Immunoprecipitation, Immunfluorsecence, and ELISA techniques.
Reagents
Use as indicated in method at 1:500 dilution, sufficient for 100 ml of working strength Ab.
Method
Results:
Western Blot Demonstration of APY03 Phosphotyrosine Specificity
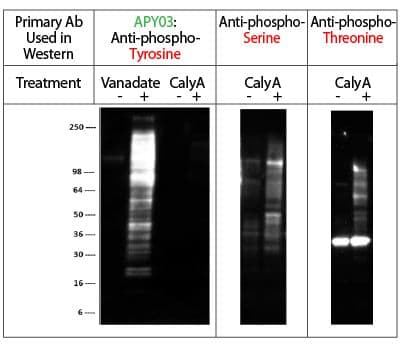
Legend: A431 cells were either treated (+) or untreated (-) with Calyculin A (CalyA: a serine/threonine phosphatase inhibitor, 50nM for 1 hour). NIH3T3 cells were either treated or untreated with H2O2-activated sodium orthovanadate (Vanadate: a specific tyrosine phosphatase inhibitor, 100 µM for 10 minutes). 16 µg of each lysate was resolved in SDS-PAGE and proteins were transferred to PVDF membrane. Membranes were probed as follows:
APY03 primary Ab: Detected tyrosine phosphorylated proteins in Vanadate-treated cells but not serine or threonine phosphorylated proteins in CalyA-treated cells.
Anti-phosphoserine & anti-phosphothreonine primary Ab: Detected serine/threonine phosphorylated proteins in CalyA-treated cells, demonstrating that phosphoserine/threonine proteins are present in CalyA treated lysates but are not detected by APY03. The anti-phosphoserine and anti-phosphothreonine Abs are from Millipore, Cat# AB1603 & AB1607 respectively.
Reagents
Use as indicated at 5 µl per IP reaction (200-500 µg total lysate per IP), sufficient for approximately 40 IP assays.
Method
Results
IP of pervanadate-treated lysates from NIH3T3 cells
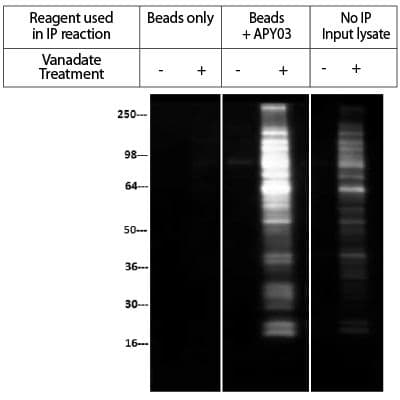
Legend: NIH3T3 cells were either treated (+) or untreated (-) with H2O2/orthovanadate (100 µM for 10min). Cell lysate was prepared in RIPA buffer and 200 µg of lysate per reaction was used for immunoprecipitation of tyrosine-phosphorylated proteins. APY03 was first bound to protein G beads and then incubated with cell lysate. For bead only control, cell lysate was incubated with protein G beads without APY03. Western blots of immunoprecipitated proteins were developed using APY03 at 1:500 dilution and CleanBlot (Thermo Scientific, #21230) as secondary antibody. No IP input lysate represents the signal from 5% of H2O2/orthovanadate treated or untreated NIH3T3 lysate. As shown in Figure 2, APY03 was able to enrich a wide range of tyrosine-phosphorylated proteins from NIH3T3 cells treated with H2O2-activated orthovanadate. No signal was detected with Protein G bead control without APY03.
Reagents
Use as indicated below at 1:500-1:1000 dilution, sufficient for 100-200 ml of working strength Ab, approx. 1000-2000 IF slides.
Method
Results
IF detection of phosphotyrosinated proteins in vanadate-treated cells
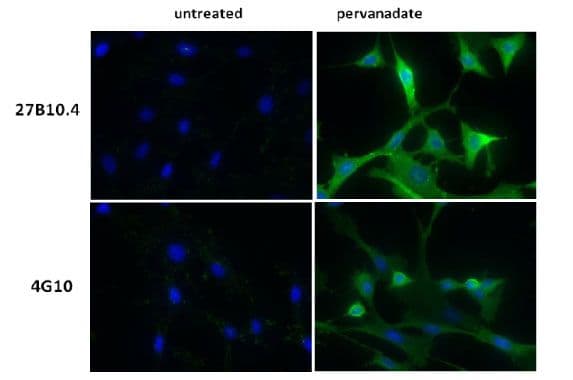
Legend: Human epidermoid carcinoma A431 cells, untreated (3A) or treated (3B) with EGF (100 ng/ml for 3 minutes), and NIH3T3, untreated (3C) or treated (3D) with H2O2-activated sodium orthovanadate (100 µM for 10 minutes), were stained as described in the method. Phosphotyrosine and nuclei were visualized in green fluorescence and blue DAPI staining, respectively.
Reagents
Use as indicated below at 1:10,000 dilution, sufficient for approximately 40,000 assays.
Method
Results
ELISA assay demonstrating specificity of APY03 for phosphotyrosine
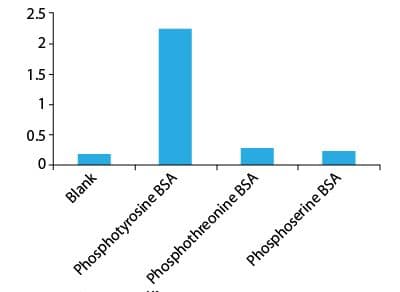
Legend: BSA was modified by conjugating with a library of phosphopeptides. 40ng of each phosphotyrosine, phosphothreonine and phosphoserine BSA were added to a individual well of a ELISA plate. APY03 at 1:10,000 dilution and goat anti mouse secondary antibody at 1:3000 were used to develop signal. Data shows that APY03 does not crossreact with phosphothreonine and phoophoserine modified BSA.
Sodium pervanadate should be made fresh and used immediately. Pervanadate is made by adding 6 µl of hydrogen peroxide to 44 µl of PBS in a microfuge tube. Add 50 µl of 200 mM sodium orthovanadate (the solution will turn yellow) and use immediately at the recommended dilutions.
Overview of PTM Detection Techniques and Methods
URL
http://lifecenter.sgst.cn/SysPTM/
What you can do
SysPTM provides a systematic and sophisticated platform for proteomic PTM research, equipped not only with a knowledge base of manually curated multi-type modification data, but also with four fully developed, in-depth data mining tools.
Literature
SysPTM: a systematic resource for proteomic research on post-translational modifications.
URL
http://140.138.144.141/~RegPhos/index.php
What you can do
RegPhos is a resource to explore the protein kinase-substrate phosphorylation networks in human and mouse.
Literature
RegPhos: a system to explore the protein kinase-substrate phosphorylation network in humans.