Fibronectin
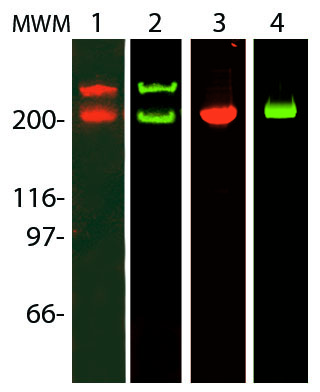
Fibronectin is a high-molecular weight (~440kDa) glycoprotein found in the extracellular matrix and in blood plasma. It is made up of two subunits that vary in size between 235-270 kDa, due to alternate splicing, (Figure 1). The secreted fibronectin dimer is a soluble protein which polymerizes to higher order fibrils in the ECM. Fibronectin plays a major role in cell adhesion, growth, migration, actin dynamics and differentiation, and it is important for processes such as wound healing and embryonic development (2). Many of these functions are mediated through fibronectin binding to integrin receptor proteins (2). Altered fibronectin expression, degradation, and organization has been associated with a number of pathologies, including cancer and fibrosis (3).
In addition to integrins, fibronectin also binds extracellular matrix components such as collagen, fibrin and heparan sulfate proteoglycans (e.g. syndecans).
Read more about assays used to study Fibronectin (Click here).
References
- Pankov R, Yamada KM . 2002. "Fibronectin at a glance". Journal of Cell Sci. 20 115: 3861-3863.
- Williams CM, Engler AJ, Slone RD, Galante LL, Schwarzbauer JE. 2008. “Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Research. 9 68: 3185-8192.
- Artym VV. Et al. 2009. ECM degradation assay for analyzing local cell invasion. Methods in molecular biology, Extracellular matrix protocols, vol. 522: 211-219.Humana Press.
Please click on a link below to read more about the products.
Rhodamine Fibronectin (Cat. # FNR01)
Engel, Leeya et al. “Extracellular matrix micropatterning technology for whole cell cryogenic electron microscopy studies.” Journal of micromechanics and microengineering : structures, devices, and systems vol. 29,11 (2019): 115018. doi:10.1088/1361-6439/ab419a
Varadaraj, Archana et al. “TGF-β triggers rapid fibrillogenesis via a novel TβRII-dependent fibronectin-trafficking mechanism.” Molecular biology of the cell vol. 28,9 (2017): 1195-1207. doi:10.1091/mbc.E16-08-0601
Mana, Giulia et al. “PPFIA1 drives active α5β1 integrin recycling and controls fibronectin fibrillogenesis and vascular morphogenesis.” Nature communications vol. 7 13546. 23 Nov. 2016, doi:10.1038/ncomms13546
Funano, S., Tanaka, N. & Tanaka, Y. Vapor-based micro/nano-partitioning of fluoro-functional group immobilization for long-term stable cell patterning. RSC Adv. 6, 96306–96313 (2016).
Comelles J. et al. 2014. Cells as active particles in asymmetric potentials: motility under external gradients. Biophys. J. 107, 1513-1522.
Steele et al., 2012. Tandem zyxin LIM sequences do not enhance force sensitive accumulation. Biochem. Biophys. Res. Commun. 422, 653–657.
Nakayama et al., 2012. Thermoresponsive Poly(N-isopropylacrylamide)-Based Block Copolymer Coating for Optimizing Cell Sheet Fabrication. Macromol. Biosci. 12, 751–760.
Tamura et al., 2012. Thermally responsive microcarriers with optimal poly(N-isopropylacrylamide) grafted density for facilitating cell adhesion/detachment in suspension culture. Acta Biomaterialia. doi:http://dx.doi.org/10.1016/j.actbio.2012.07.006.
Steward et al., 2011. Mechanical stretch and shear flow induced reorganization and recruitment of fibronectin in fibroblasts. Sci. Rep. 1, 147. doi: 10.1038/srep00147.
Nagase et al., 2011. Thermo-Responsive Polymer Brushes as Intelligent Biointerfaces: Preparation via ATRP and Characterization. Macromol Biosci. 11, 300-309.
Nagase K et al. (2010). Thermo-Responsive Polymer Brushes as Intelligent Biointerfaces: Preparation via ATRP and Characterization.. Macromol Biosci.
Robinson, E. E., Foty, R. A. and Corbett, S. A. (2004). Fibronectin matrix assembly regulates α5β1-mediated cell cohesion. Mol. Biol. Cell 15, 973-981.
Brock, A., Chang, E., Ho, C. C., LeDuc, P., Jiang, X., Whitesides, G. M. and Ingber, D. E. (2003). Geometric determinants of directional cell motility revealed using microcontact printing. Langmuir 19, 1611-1617.
Fibronectin (Green fluorescent, HiLyte™ 488) (Cat. # FNR02)
Torr E.E. et al. 2015. Myofibroblasts exhibit enhanced fibronectin assembly that is intrinsic to their contractile phenotype. J. Biol. Chem. 290, 6951-6961.
Jacob A., et al. 2013. Rab40b regulates MMP2 and MMP9 trafficking during invadopodia formation and breast cancer cell invasion. J. Cell Sci. doi: 10.1242/jcs.126573
Lively and Schlichter, 2013. The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion. J. Neuroinflammation. 10:75.
Fibronectin (Biotinylated) (Cat. # FNR03)
Varadaraj, Archana et al. “TGF-β triggers rapid fibrillogenesis via a novel TβRII-dependent fibronectin-trafficking mechanism.” Molecular biology of the cell vol. 28,9 (2017): 1195-1207. doi:10.1091/mbc.E16-08-0601
N.M. Rodriguez et al., 2014. Micropatterned multicolor dynamically adhesive substrates to control cell adhesion and multicellular organization. Langmuir. DOI: 10.1021/la404037s.
Question 1: Can I coat cells with fluorescent ECM proteins?
Answer 1: Yes, cells can be coated with fluorescently-labeled ECM proteins such as fibronectin. Example protocols are found in Pallis et al., 1997 (Peripheral Blood Lymphocyte Binding to a Soluble FITC-Fibronectin Conjugate. Cytometry. 28, 157-164) and Huveneers et al., 2008 (Binding of soluble fibronectin to integrin a5b1 – link to focal adhesion redistribution and contractile shape. J. Cell Sci. 121, 2452-2462). Briefly, cells are incubated with a low concentration of soluble fluorescently-labeled fibronectin in an appropriate buffer. Low concentrations of fibronectin are recommended to minimize non-specific binding. The cells/fibronectin mixture is incubated in the dark for 30 min at room temperature. Others have done the incubation at 4°C for 1 hour. The cells are then rinsed in an appropriate buffer, resuspended in the same buffer, and then the coating/binding of ECM proteins can be quantified by flow cytometry or the coated cells can be used experimentally.
-
-
- Fibronectin (Fluorescent Red/Orange HiLyte Fluor™ 555 Labeled) FNR05Learn More
Fibronectin (Fluorescent Red/Orange HiLyte Fluor™ 555 Labeled)
- Fibronectin (Green fluorescent, HiLyte 488) FNR02Learn MoreFibronectin (Green fluorescent, HiLyte 488)
-
