Small GTPase activators & inhibitors
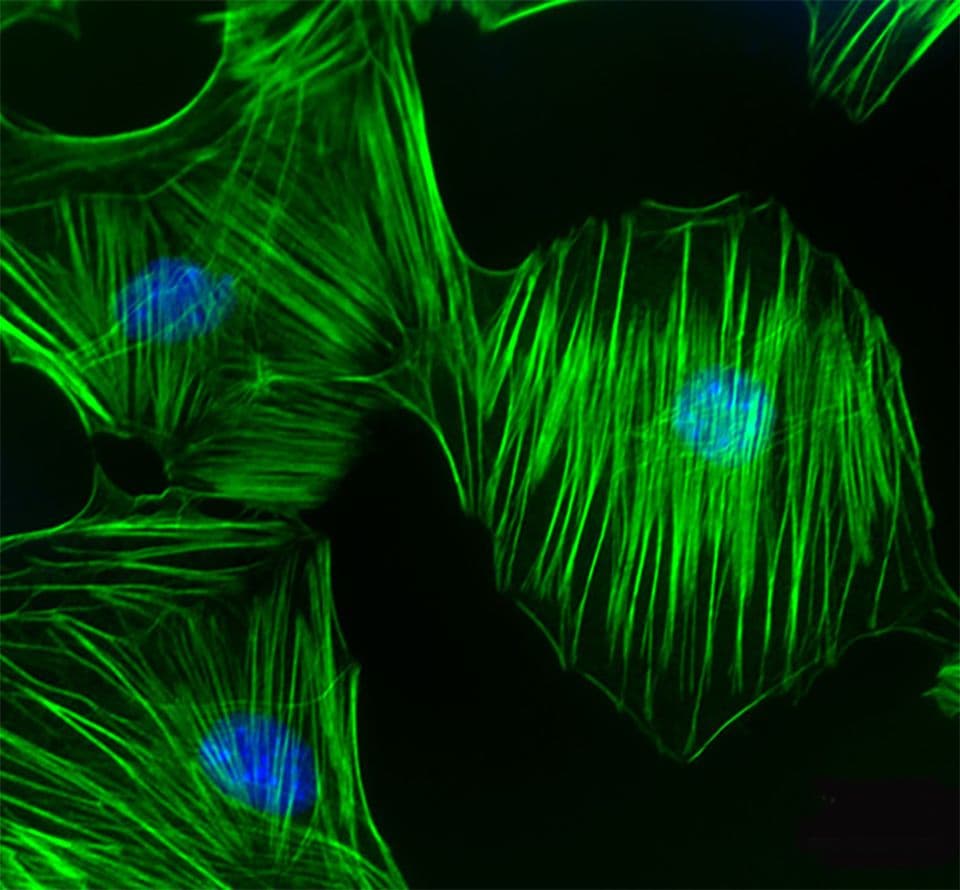
The G-Switch™ line provides a comprehensive portfolio of cell-permeable GTPase activators and inhibitors specifically designed to modulate GTPase signaling pathways in live cells.
The product line features highly selective Rho GTPase inhibitors and activators that act directly and irreversibly on endogenous GTPases. Unlike traditional methods such as siRNA knockdown or overexpression of mutant constructs like Rho Q63L, these reagents offer a more precise and efficient means of manipulating GTPase activity.
The G-Switch™ line provides a comprehensive portfolio of cell-permeable GTPase activators and inhibitors specifically designed to modulate GTPase signaling pathways in live cells.
The product line features highly selective Rho GTPase inhibitors and activators that act directly and irreversibly on endogenous GTPases. Unlike traditional methods such as siRNA knockdown or overexpression of mutant constructs like Rho Q63L, these reagents offer a more precise and efficient means of manipulating GTPase activity.