Actin & Actin Binding Proteins
HTS assays for actin and actin binding proteins (CytoDYNAMIX Screen™ 20 series)
Actin and actin binding proteins can be used as targets in a drug screen to develop the next generation of drugs to treat cancer, neurological disorders, inflammation, and infectious diseases. Many actin isotypes and actin binding proteins such as WASP, cofilin, and profilin can be targeted in a drug screen based on the Biochem Kit series of assays. These assays utilize modified actins such as pyrene or biotin conjugates to report on the effects of a drug. If you would like more information about setting up one of these assays as a drug screen, please contact our technical support department.
To study polymerization of non-labeled actin, the Actin Polymerization Assay (Cat. # BK003) can be purchased along with your actin of choice (e.g., cardiac, Cat. # AD99; smooth, Cat. # AS99; or non-muscle actin, Cat. # APHL99). Cytoskeleton's Actin Polymerization Assay comes with pyrene labeled muscle actin (Cat. # AP05) that is used as a reporter molecule for polymerization of the non-labeled actin of choice.
To discuss Actin Binding ELISA-type and Actin Filament Filter Based Assays contact our technical support department.
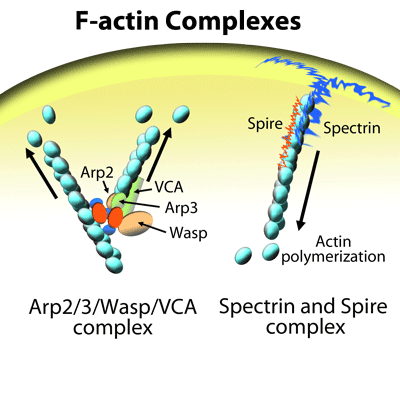
Cytoskeleton's actin products have been cited hundreds of times over the past 18 years. A select few are described here, for more citations on individual products please use the "Citations" tab on each individual product page.
Actin protein (>99% pure): rabbit skeletal muscle (Cat. # AKL99) |
| Windhorst S, Kalinina T, Schmid K, Blechner C, Kriebitzsch N, Hinsch R, Chang L, Herich L, Schumacher U, Mayr GW. (2010). Functional role of inositol-1,4,5-trisphosphate-3-kinase-A for motility of malignant transformed cells. Int J Cancer. |
| Arora, P. D., Glogauer, M., Kapus, A., Kwiatkowski, D. J. and McCulloch, C. A. (2004). Gelsolin mediates collagen phagocytosis through a rac-dependent step. Mol. Biol. Cell 15, 588-599. |
| Ishikawa, T., Cheng, N., Liu, X., Korn, E. D. and Steven, A. C. (2004). Subdomain organization of the Acanthamoeba myosin IC tail from cryo-electron microscopy. Proc. Natl. Acad. Sci. U. S. A. 101, 12189-12194. |
| Balcer, H. I., Goodman, A. L., Rodal, A. A., Smith, E., Kugler, J., Heuser, J. E. and Goode, B. L. (2003). Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr. Biol. 13, 2159-2169. |
| Loomis, P. A., Zheng, L., Sekerkova, G., Changyaleket, B., Mugnaini, E. and Bartles, J. R. (2003). Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J. Cell Biol. 163, 1045-1055. |
| Upadhyaya, A., Chabot, J. R., Andreeva, A., Samadani, A. and van Oudenaarden, A. (2003). Probing polymerization forces by using actin-propelled lipid vesicles. Proc. Natl. Acad. Sci. U. S. A. 100, 4521-4526. |
| Humphries, C. L., Balcer, H. I., D'Agostino, J. L., Winsor, B., Drubin, D. G., Barnes, G., Andrews, B. J. and Goode, B. L. (2002). Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. J. Cell Biol. 159, 993-1004. |
| Sagot, I., Rodal, A. A., Moseley, J., Goode, B. L. and Pellman, D. (2002). An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 4, 626-631. |
| Engqvist-Goldstein, A. E., Warren, R. A., Kessels, M. M., Keen, J. H., Heuser, J. and Drubin, D. G. (2001). The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. J. Cell Biol. 154, 1209-1223. |
| McGhie, E. J., Hayward, R. D. and Koronakis, V. (2001). Cooperation between actin-binding proteins of invasive Salmonella: SipA potentiates SipC nucleation and bundling of actin. EMBO J. 20, 2131-2139. |
Actin Polymerization Biochem Kit (fluorescence format): rabbit skeletal muscle actin (Cat. # BK003) |
| Takamiya, R., Takahashi, M., Park, Y. S., Tawara, Y., Fujiwara, N., Miyamoto, Y., Gu, J., Suzuki, K. and Taniguchi, N. (2005). Overexpression of mutated Cu,Zn-SOD in neuroblastoma cells results in cytoskeletal change. Am. J. Physiol. 288, C253-259. |
| Kumar, N., Tomar, A., Parrill, A. L. and Khurana, S. (2004). Functional dissection and molecular characterization of calcium-sensitive actin-capping and actin-depolymerizing sites in villin. J. Biol. Chem. 279, 45036-45046. |
| Fontao, L., Geerts, D., Kuikman, I., Koster, J., Kramer, D. and Sonnenberg, A. (2001). The interaction of plectin with actin: evidence for cross-linking of actin filaments by dimerization of the actin-binding domain of plectin. J. Cell Sci. 114, 2065-2076. |
| Zhai, L., Zhao, P., Panebra, A., Guerrerio, A. L. and Khurana, S. (2001). Tyrosine phosphorylation of villin regulates the organization of the actin cytoskeleton. J. Biol. Chem. 276, 36163-36167. |
| Blader, I. J., Cope, M. J., Jackson, T. R., Profit, A. A., Greenwood, A. F., Drubin, D. G., Prestwich, G. D. and Theibert, A. B. (1999). GCS1, an Arf guanosine triphosphatase-activating protein in Saccharomyces cerevisiae, is required for normal actin cytoskeletal organization in vivo and stimulates actin polymerization in vitro. Mol. Biol. Cell 10, 581-596. |
Cofilin 1 protein: human recombinant (Cat. # CF01) |
| van der Gucht, J., Paluch, E., Plastino, J. and Sykes, C. (2005). Stress release drives symmetry breaking for actin-based movement. Proc. Natl. Acad. Sci. U. S. A. 102, 7847-7852. |
| Loomis, P. A., Zheng, L., Sekerkova, G., Changyaleket, B., Mugnaini, E. and Bartles, J. R. (2003). Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J. Cell Biol. 163, 1045-1055. |
| Vignjevic, D., Yarar, D., Welch, M. D., Peloquin, J., Svitkina, T. and Borisy, G. G. (2003). Formation of filopodia-like bundles in vitro from a dendritic network. J. Cell Biol. 160, 951-962. |
| Yokoo, T., Toyoshima, H., Miura, M., Wang, Y., Iida, K. T., Suzuki, H., Sone, H., Shimano, H., Gotoda, T., Nishimori, S. et al. (2003). p57Kip2 regulates actin dynamics by binding and translocating LIM-kinase 1 to the nucleus. J. Biol. Chem. 278, 52919-52923. |
| Idrissi, F. Z., Wolf, B. L. and Geli, M. I. (2002). Cofilin, but not profilin, is required for myosin-I-induced actin polymerization and the endocytic uptake in yeast. Mol. Biol. Cell 13, 4074-4087. |
Question 1: What format is best when designing an assay that measures actin polymerization modulated by an actin binding protein (ABP)?
Answer 1: The best way to measure the effects of an ABP on actin polymerization is to use Cytoskeleton’s actin polymerization biochem kit (Cat. # BK003). The kit uses pyrene-labeled actin to visualize actin polymerization (and depolymerization, if desired). This kit can be adapted to multiple actin sources (muscle, non-muscle, cardiac) and is ideal for discovering and/or characterizing ABPs in the form of compounds, purified proteins or tissue extracts. Changes in one or more of the three stages of polymerization (nucleation, growth or steady state) can be evaluated with this kit. The kit comes with all of the necessary reagents and buffers to perform actin polymerization assays. Click on the Citations tab for references of where these kits were used in research projects.
Question 2: Do you have an actin monomer binding assay?
Answer 2: To examine compounds, proteins or tissue extracts that bind to actin monomers, 96-well plates with streptavidin-coated wells can be complexed with biotinylated actin (Cat. # AB07) to create an actin monomer capture protein. Then the compound, protein or extract can be incubated with the capture proteins and binding can be visualized by an HRP detection system using an antibody (or a tag can be conjugated to the test protein) against the compound or protein of interest. Keep in mind that buffer composition is also important as it will need to be low salt (approx 50 mM) and contain CaCl2 (or other divalent cation) and ATP to maintain actin monomer stability. For an example of a compatible buffer, see our recipe for general actin buffer (Cat. # BSA01-001). Alternatively, the protein of interest could be tagged with GST (avoid reducing agents) and conjugated to the plate and then incubated with biotinylated actin and visualized with HRP-conjugated streptavidin.
For more information, click on Documents and see the datasheet, or for Technical Support, e-mail tservice@cytoskeleton.com.
- Actin Polymerization Biochem Kit (fluorescence format): rabbit skeletal muscle actin BK003Learn MoreActin Polymerization Biochem Kit (fluorescence format): rabbit skeletal muscle actin
- Actin protein ( >99% pure): human platelet APHL99Learn MoreActin protein (>99% pure): human platelet
- Actin protein ( >99% pure): rabbit skeletal muscle AKL99Learn MoreActin protein (>99% pure): rabbit skeletal muscle
- Actin protein (>99% pure): bovine cardiac muscle AD99Learn MoreActin protein (>99% pure): bovine cardiac muscle
- Actin protein (>99% pure): chicken gizzard muscle AS99Learn MoreActin protein (>99% pure): chicken gizzard muscle
-
- Actin protein (pyrene labeled): rabbit skeletal muscle AP05Actin protein (pyrene labeled): rabbit skeletal muscle Learn More
-
-
- Gelsolin protein: Homo sapiens recombinant HPG6Learn MoreGelsolin protein: Homo sapiens recombinant
- Profilin 1 protein: Untagged, human recombinant PR02Learn MoreProfilin 1 protein: Untagged, human recombinant
