Proteins
Cytoskeleton is the pre-eminent supplier of Rho and Ras family small G-proteins (SGPs). The proteins are available in wild-type, constitutively active and dominant negative forms. These products have been fully QC'd for high activity and purity. For more information click on the datasheet below.
Apart from the purified proteins, Cytoskeleton also provides some innovative kits for studying these proteins in cells and in vitro, see related products below for more information.
Related Products
For more information view our datasheets below, or contact Technical Support at tservice@cytoskeleton.com .
Cytoskeleton's products have been cited hundreds of times over the past 18 years. A select few are described here, for more citations on individual products please use the "Citations" tab on each individual product page.
Cdc42 protein: GST tagged: human dominant negative (Cat. # C17G01) |
| Ezratty, E. J., Partridge, M. A. and Gundersen, G. G. (2005). Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7, 581-590. |
| Gomes, E. R., Jani, S. and Gundersen, G. G. (2005). Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451-463. |
| Burakov, A., Nadezhdina, E., Slepchenko, B. and Rodionov, V. (2003). Centrosome positioning in interphase cells. J. Cell Biol. 162, 963-969. |
| Sokac, A. M., Co, C., Taunton, J. and Bement, W. (2003). Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat. Cell Biol. 5, 727-732. |
| Zhang, X. F., Schaefer, A. W., Burnette, D. T., Schoonderwoert, V. T. and Forscher, P. (2003). Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron 40, 931-944. |
| Palazzo, A. F., Joseph, H. L., Chen, Y. J., Dujardin, D. L., Alberts, A. S., Pfister, K. K., Vallee, R. B. and Gundersen, G. G. (2001). Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr. Biol. 11, 1536-1541. |
| Fenteany, G., Janmey, P. A. and Stossel, T. P. (2000). Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr. Biol. 10, 831-838. |
| Kalinec, F., Zhang, M., Urrutia, R. and Kalinec, G. (2000). Rho GTPases mediate the regulation of cochlear outer hair cell motility by acetylcholine. J. Biol. Chem. 275, 28000-28005. |
Cdc42 protein: His tagged: human constitutively active (Cat. # C6101) |
| Zhang, X. F., Schaefer, A. W., Burnette, D. T., Schoonderwoert, V. T. and Forscher, P. (2003). Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron 40, 931-944. |
Cdc42 protein: His tagged: human wild type (Cat. # CD01) |
| Nevins, A. K. and Thurmond, D. C. (2005). A direct interaction between Cdc42 and vesicle-associated membrane protein 2 regulates SNARE-dependent insulin exocytosis. J. Biol. Chem. 280, 1944-1952. |
| Kulkarni, S., Goll, D. E. and Fox, J. E. (2002). Calpain cleaves RhoA generating a dominant-negative form that inhibits integrin-induced actin filament assembly and cell spreading. J. Biol. Chem. 277, 24435-24441. |
Cdc42 protein: GSt tagged: human wid type (Cat. # CDG01) |
| Lesser, C. F. and Miller, S. I. (2001). Expression of microbial virulence proteins in Saccharomyces cerevisiae models mammalian infection. EMBO J. 20, 1840-1849. |
| Slater, S. J., Seiz, J. L., Stagliano, B. A. and Stubbs, C. D. (2001). Interaction of protein kinase C isozymes with Rho GTPases. Biochemistry 40, 4437-4445. |
Rho GAP protein:GST tagged: Homo sapiens recombinant (Cat. # GAP01) |
| Li, X., Bu, X., Lu, B., Avraham, H., Flavell, R. A. and Lim, B. (2002). The hematopoiesis-specific GTP-binding protein RhoH is GTPase deficient and modulates activities of other Rho GTPases by an inhibitory function. Mol. Cell. Biol. 22, 1158-1171. |
Rho GAP protein (catalytic domain): GST tagged: Homo sapiens recombinant (Cat. # GAS01) |
| Li, X., Bu, X., Lu, B., Avraham, H., Flavell, R. A. and Lim, B. (2002). The hematopoiesis-specific GTP-binding protein RhoH is GTPase deficient and modulates activities of other Rho GTPases by an inhibitory function. Mol. Cell. Biol. 22, 1158-1171. |
Rac1 protein: GST tagged: human dominant negative (Cat. # R17G01) |
| Ezratty, E. J., Partridge, M. A. and Gundersen, G. G. (2005). Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7, 581-590. |
| Grabham, P. W., Reznik, B. and Goldberg, D. J. (2003). Microtubule and Rac 1-dependent F-actin in growth cones. J. Cell Sci. 116, 3739-3748. |
| Zhang, X. F., Schaefer, A. W., Burnette, D. T., Schoonderwoert, V. T. and Forscher, P. (2003). Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron 40, 931-944. |
| Storey, N. M., O'Bryan, J. P. and Armstrong, D. L. (2002). Rac and Rho mediate opposing hormonal regulation of the ether-a-go-go-related potassium channel. Curr. Biol. 12, 27-33. |
| Palazzo, A. F., Joseph, H. L., Chen, Y. J., Dujardin, D. L., Alberts, A. S., Pfister, K. K., Vallee, R. B. and Gundersen, G. G. (2001). Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr. Biol. 11, 1536-1541. |
| Kalinec, F., Zhang, M., Urrutia, R. and Kalinec, G. (2000). Rho GTPases mediate the regulation of cochlear outer hair cell motility by acetylcholine. J. Biol. Chem. 275, 28000-28005. |
Rac1 protein: His tagged: human constitutively active (Cat. # R6101) |
| Storey, N. M., O'Bryan, J. P. and Armstrong, D. L. (2002). Rac and Rho mediate opposing hormonal regulation of the ether-a-go-go-related potassium channel. Curr. Biol. 12, 27-33. |
RhoA protein: His tagged: human constitutively active (Cat. # R6301) |
| Zhang, X. F., Schaefer, A. W., Burnette, D. T., Schoonderwoert, V. T. and Forscher, P. (2003). Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron 40, 931-944. |
| Gallo, G., Yee, H. F., Jr. and Letourneau, P. C. (2002). Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J. Cell Biol. 158, 1219-1228. 34 |
| Storey, N. M., O'Bryan, J. P. and Armstrong, D. L. (2002). Rac and Rho mediate opposing hormonal regulation of the ether-a-go-go-related potassium channel. Curr. Biol. 12, 27-33. |
Rac1 protein: His tagged: human wild type (Cat. # RC01) |
| Kulkarni, S., Goll, D. E. and Fox, J. E. (2002). Calpain cleaves RhoA generating a dominant-negative form that inhibits integrin-induced actin filament assembly and cell spreading. J. Biol. Chem. 277, 24435-24441. |
Rac2 protein: His tagged: human wild type (Cat. # RC02) |
| Kulkarni, S., Goll, D. E. and Fox, J. E. (2002). Calpain cleaves RhoA generating a dominant-negative form that inhibits integrin-induced actin filament assembly and cell spreading. J. Biol. Chem. 277, 24435-24441. |
Rac1 protein: GST tagged: human wild type (Cat. # RCG01) |
| Teckchandani, A. M., Panetti, T. S. and Tsygankov, A. Y. (2005). c-Cbl regulates migration of v-Abl-transformed NIH 3T3 fibroblasts via Rac1. Exp. Cell Res. 307, 247-258. |
| Grabham, P. W., Reznik, B. and Goldberg, D. J. (2003). Microtubule and Rac 1-dependent F-actin in growth cones. J. Cell Sci. 116, 3739-3748. |
| Li, X., Bu, X., Lu, B., Avraham, H., Flavell, R. A. and Lim, B. (2002). The hematopoiesis-specific GTP-binding protein RhoH is GTPase deficient and modulates activities of other Rho GTPases by an inhibitory function. Mol. Cell. Biol. 22, 1158-1171. |
| Lesser, C. F. and Miller, S. I. (2001). Expression of microbial virulence proteins in Saccharomyces cerevisiae models mammalian infection. EMBO J. 20, 1840-1849. |
| Slater, S. J., Seiz, J. L., Stagliano, B. A. and Stubbs, C. D. (2001). Interaction of protein kinase C isozymes with Rho GTPases. Biochemistry 40, 4437-4445. |
RhoA protein: His tagged: human wild type (Cat. # RH01) |
| Lee, J. H., Katakai, T., Hara, T., Gonda, H., Sugai, M. and Shimizu, A. (2004). Roles of p-ERM and Rho-ROCK signaling in lymphocyte polarity and uropod formation. J. Cell Biol. 167, 327-337. |
| Kulkarni, S., Goll, D. E. and Fox, J. E. (2002). Calpain cleaves RhoA generating a dominant-negative form that inhibits integrin-induced actin filament assembly and cell spreading. J. Biol. Chem. 277, 24435-24441. |
RhoA protein: GST tagged: human wild type (Cat. # RHG01) |
| Teckchandani, A. M., Panetti, T. S. and Tsygankov, A. Y. (2005). c-Cbl regulates migration of v-Abl-transformed NIH 3T3 fibroblasts via Rac1. Exp. Cell Res. 307, 247-258. |
| Patil, S. B., Pawar, M. D. and Bitar, K. N. (2004). Phosphorylated HSP27 essential for acetylcholine-induced association of RhoA with PKCα. Am. J. Physiol. 286, G635-644. |
| Yamashita, T. and Tohyama, M. (2003). The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat. Neurosci. 6, 461-467. |
| Lesser, C. F. and Miller, S. I. (2001). Expression of microbial virulence proteins in Saccharomyces cerevisiae models mammalian infection. EMBO J. 20, 1840-1849. |
| Slater, S. J., Seiz, J. L., Stagliano, B. A. and Stubbs, C. D. (2001). Interaction of protein kinase C isozymes with Rho GTPases. Biochemistry 40, 4437-4445. |
Question 1: Can I use the mutant Rho proteins for micro-injection studies?
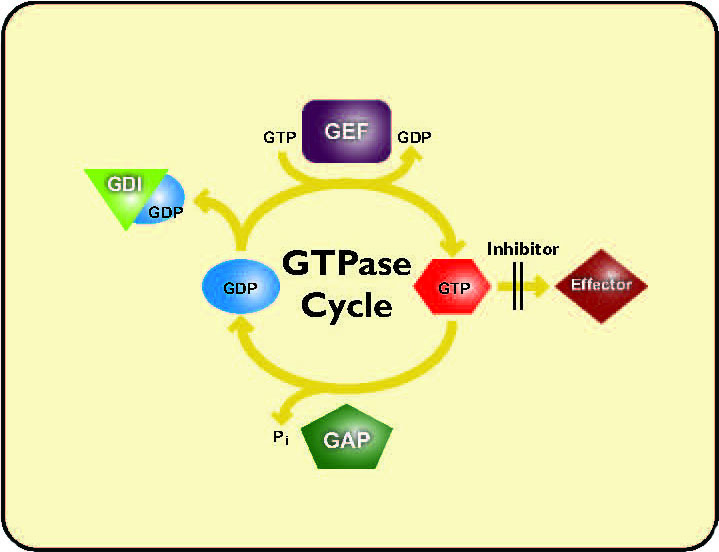
Answer 1: Yes, Cytoskeleton’s wide selection of wild-type, dominant-negative and constitutively-active purified small G-proteins can be used for micro-injection studies to examine G-protein activation in cells. Alternatively, cell phenotypes and G-protein activation levels can be assessed with our G-switch™ line of small G-protein tools. The G-switch™ line includes direct and indirect activators of the Rho family of proteins. Activators include the indirect Rho activator calpeptin (Cat. # CN01), indirect Rac/Cdc42 activator EGF (Cat. # CN02), a specific and direct Rho activator (Cat. # CN03) and a direct Rho/Rac/Cdc42 activator (Cat. # CN04). The direct GTPase activators (Cat. # CN03 and CN04) are ideal for examining G-protein activation since they act rapidly on the endogenous target protein (<3 hours), thus avoiding non-specific activation of other pathways. The activated state of GTPases is maintained for hours, providing an ample experimental window for studying GTPase activation or inhibition of the activated protein.
Question 2: Can the PAK-PBD and Rhotekin-RBD be used to localize active small G-proteins in cells?
Answer 2: Yes, the GST-tagged PAK-PBD (Cat. # PAK01) and rhotekin-RBD (Cat. # RT01) proteins can be used to identify activated small G-proteins in fixed and permeabilized cells. Examples of this technique are reported in Berdeaux et al., 2004 (Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J. Cell Biol. 166, 317-323) and Zhao et al., 2007 (Force activates smooth muscle -actin promoter activity through the Rho signaling pathway. J. Cell Sci. 120, 1801-1809). Briefly, cells are grown on glass coverslips, treated with control and experimental conditions, fixed, permeabilized and incubated with PAK-PBD or Rhotekin-RBD proteins. Localization of the bound proteins is accomplished by immunocytochemical detection of the GST tag with an anti-GST antibody that will allow localization of the activated small G-proteins bound by PAK-PBD or Rhotekin-RBD. Cytoskeleton has not verified this technique as a viable and accurate means of localizing activated small G-proteins and because it is not a widely utilized technique, we cannot confirm that this procedure achieves an accurate representation of activated GTPases.
- ARNO GEF Protein (Sec7 Exchange Domain, aa31-267, 6xHis tag) CS-GE07Learn MoreARNO GTP exchange Protein (Sec7 domain)
- Cdc42 protein: GST tagged: human dominant negative C17G01Learn MoreCdc42 protein: GST tagged: human dominant negative
- Cdc42 protein: GSt tagged: human wid type CDG01Learn MoreCdc42 protein: GSt tagged: human wid type
- Cdc42 protein: His tagged: human constitutively active C6101Learn MoreCdc42 protein: His tagged: human constitutively active
- Cdc42 protein: His tagged: human wild type CD01Learn MoreCdc42 protein: His tagged: human wild type
- H-Ras protein: His tagged: human wild type RS01Learn MoreH-Ras protein: His tagged: human wild type
- K-Ras4B G12C mutated protein (Human recombinant, 6xHis-tag) CS-RS14Learn More
K-Ras4B G12C mutated protein (Human recombinant, 6xHis-tag)
- K-Ras4B Mutant Protein: G12D+D38A (human recombinant with 6xHis-tag) CS-RS12Learn More
K-Ras4B Mutant Protein: G12D+D38A (human recombinant with 6xHis-tag)
- K-Ras4B Mutant Protein: G12D+I36N (human recombinant with 6xHis-tag) CS-RS11K-Ras4B Mutant Protein: G12D+I36N (human recombinant with 6xHis-tag) Learn More
- K-Ras4B Mutant Protein: Q61P (human recombinant with 6xHis-tag) CS-RS09Learn More
K-Ras4B Mutant Protein: Q61P (human recombinant with 6xHis-tag)
- K-Ras4B Mutant Protein: R135A (human recombinant with 6xHis-tag) CS-RS10Learn More
K-Ras4B Mutant Protein: R135A (human recombinant with 6xHis-tag)
- K-Ras4B Protein: G12D (Human recombinant, 6xHis-tag) CS-RS13Learn MoreK-Ras4B Protein: G12D (Human recombinant, 6xHis-tag)
- K-Ras4B Protein: G12V (Human recombinant) CS-RS04Learn MoreK-Ras4B Protein: G12V (Human recombinant)
- K-Ras4B Protein: G13D (Human recombinant, 6xHis-tag) CS-RS06K-Ras4B Protein: G13D (Human recombinant, 6xHis-tag) Learn More
- K-Ras4B Protein: G13S (Human recombinant, 6xHis-tag) CS-RS07K-Ras4B Protein: G13S (Human recombinant, 6xHis-tag) Learn More
- K-Ras4B Protein: K128A (Human recombinant, 6xHis-tag) CS-RS08Learn MoreK-Ras4B Protein: K128A (Human recombinant, 6xHis-tag)
-
- N-Ras Protein: wild-type (Human recombinant) CS-RS02Learn MoreN-Ras Protein: wild-type (Human recombinant)
- PAK-PBD beads (binds active Rac/Cdc42 proteins) PAK02Learn MorePAK-PBD beads (binds active Rac/Cdc42 proteins)
-
-
- Rac1 protein: GST tagged: human dominant negative R17G01Learn MoreRac1 protein: GST tagged: human dominant negative
- Rac1 protein: GST tagged: human wild type RCG01Learn MoreRac1 protein: GST tagged: human wild type
- Rac1 protein: His tagged: human constitutively active R6101Rac1 protein: His tagged: human constitutively active Learn More
-
-
-
- Rap1b protein: His tagged: human wild type RR02Learn MoreRap1b protein: His tagged: human wild type
- RAPGEF5 Protein: Ras association and exchange domain (57– 580) wild type. (Human recombinant, GST tagged) CS-GE09Learn MoreRAPGEF5 Protein: Ras association and exchange domain (57– 580) wild type. (Human recombinant, GST tagged)
- RasGRF1 GEF Protein (Cdc25 Exchange Domain, aa1038-1270, MBP tag) CS-GE03Learn MoreRasGRF1 GTP exchange Protein (Cdc25 domain)
- Rho GAP protein (catalytic domain): GST tagged: Homo sapiens recombinant GAS01Learn MoreRho GAP protein (catalytic domain): GST tagged: Homo sapiens recombinant
- Rho GAP protein:GST tagged: Homo sapiens recombinant GAP01Learn MoreRho GAP protein:GST tagged: Homo sapiens recombinant
- RhoA protein: GST tagged: human wild type RHG01Learn MoreRhoA protein: GST tagged: human wild type
- RhoA protein: His tagged: human constitutively active R6301RhoA protein: His tagged: human constitutively active Learn More
-
-
- Rhotekin-RBD beads (binds active Rho proteins) RT02Learn MoreRhotekin-RBD beads (binds active Rho proteins)
- Rhotekin-RBD protein (binds active Rho proteins) RT01Learn MoreRhotekin-RBD protein (binds active Rho proteins)
- SOS1 exchange domain (564-1049) protein : GST-tagged (Human recombinant) CS-GE10Learn More
SOS1 exchange domain (564-1049) protein : GST-tagged (Human recombinant)
- SOS1 Protein (ExD Exchange Domain, aa564-1049, 6xHis tag) GE02Learn MoreSOS1 Protein (ExD Exchange Domain, aa564-1049)
