Skeletal myosin II is a motor protein essential for muscle contraction, converting chemical energy from ATP into mechanical force to slide actin filaments. It plays a central role in generating the tension required for voluntary movement, making it critical for locomotion and posture maintenance.
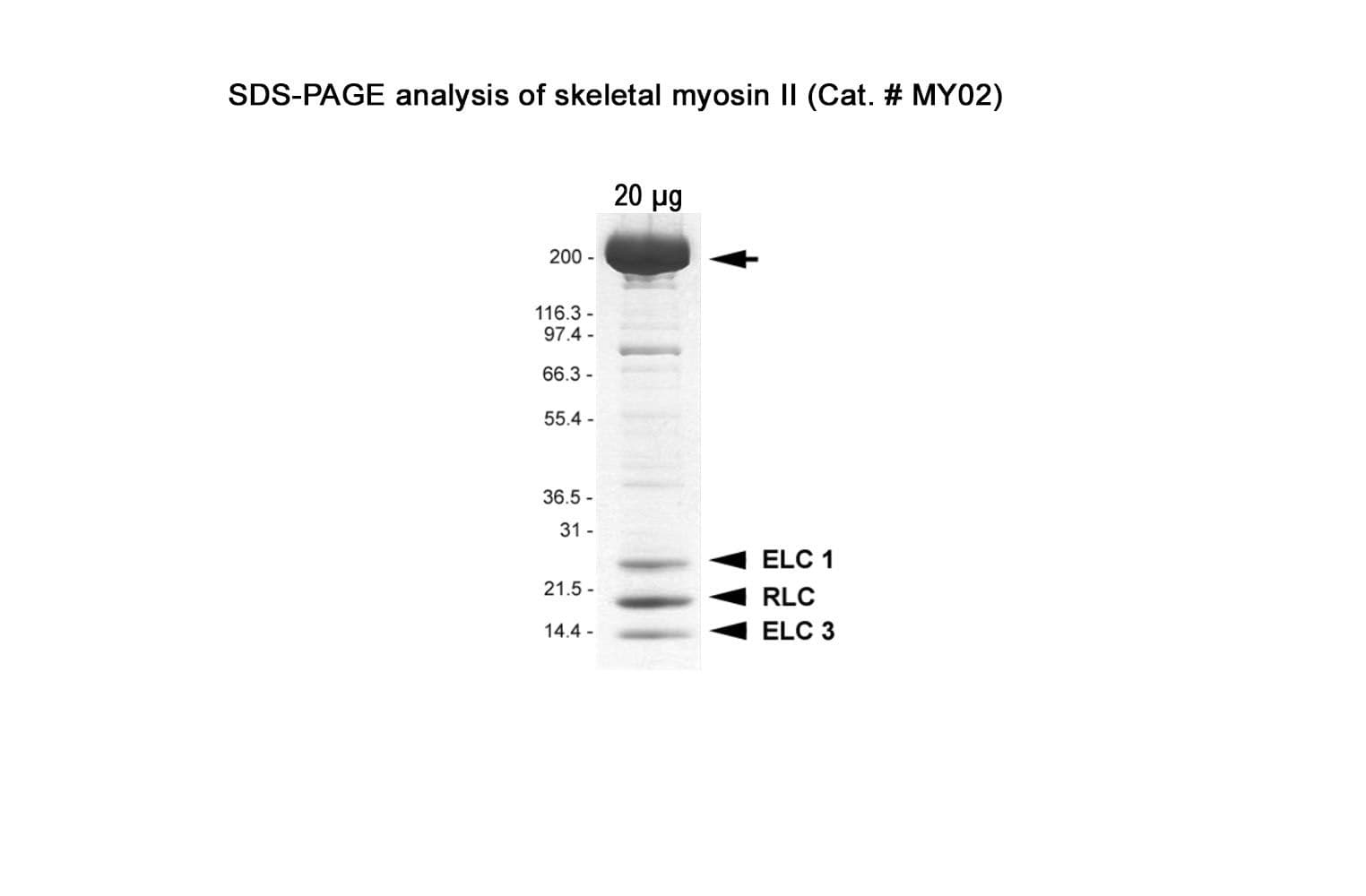
Native myosin II protein is purified from rabbit skeletal muscle. The full-length myosin II heavy chain protein (~200 kDa) is purified with its essential light chains (ELC: ~25 & 17 kDa) and regulatory light chains (RLC: ~20 kDa).
Protein purity is assessed by scanning densitometry of Coomassie Blue-stained protein on a4-20% polyacrylamide gel. Purity is determined to be >90% pure.
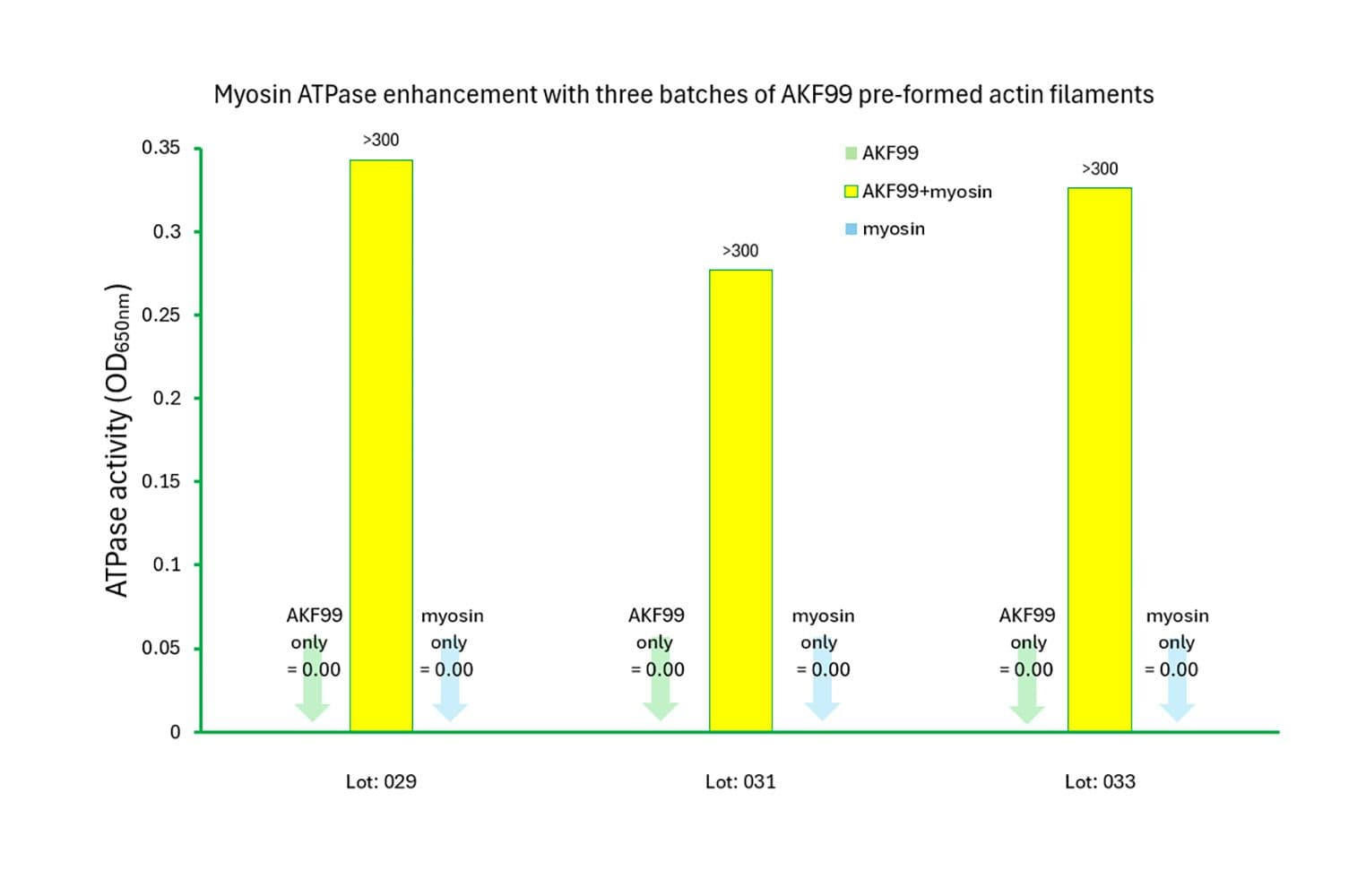
The biological activity of rabbit myosin II is measured by its rate of F-actin–activated ATP hydrolysis. This is assessed using a standard in vitro F-actin ATPase assay. Quality control requires that, in the presence of F-actin, the ATP hydrolysis rate must be at least 10 times greater than in its absence, and the actin-activated ATPase activity must exceed 300 nmol/min/mg of myosin under the experimental conditions (see datasheet).
Cat. #MY02