Cardiac myosin is a specialized motor protein that drives heart muscle contraction by converting chemical energy from ATP into mechanical force. Modulation of cardiac myosin activity is an active area of therapeutic interest.
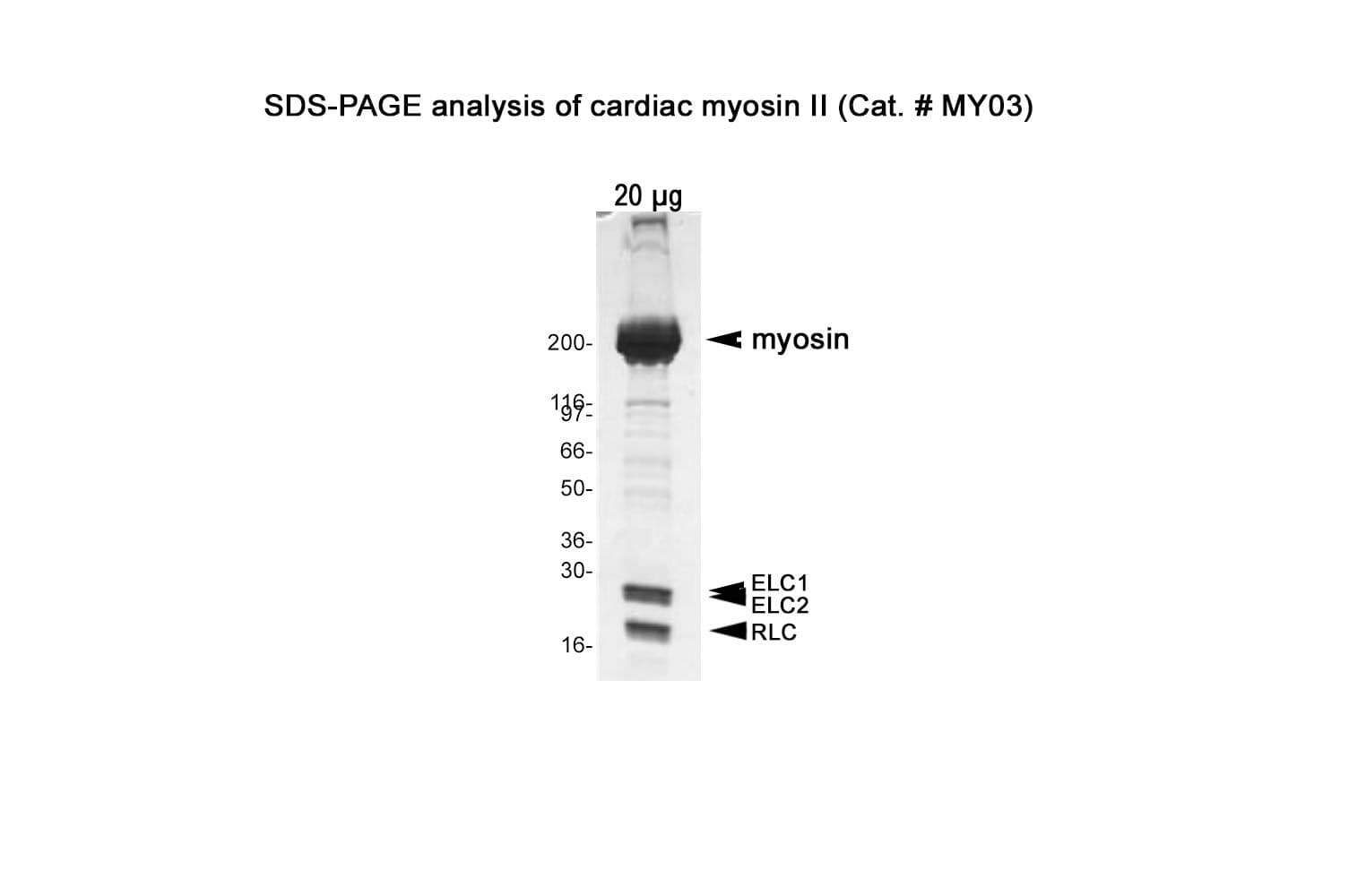
Native cardiac myosin protein is purified from bovine cardiac muscle. The preparation contains the full-length myosin heavy chain (~200 kDa) along with its associated essential light chains (~25 kDa and ~17 kDa) and regulatory light chains (~20 kDa).
Protein purity is assessed by scanning densitometry of Coomassie Blue-stained protein on a4-20% polyacrylamide gel. Purity is determined to be >90% pure.
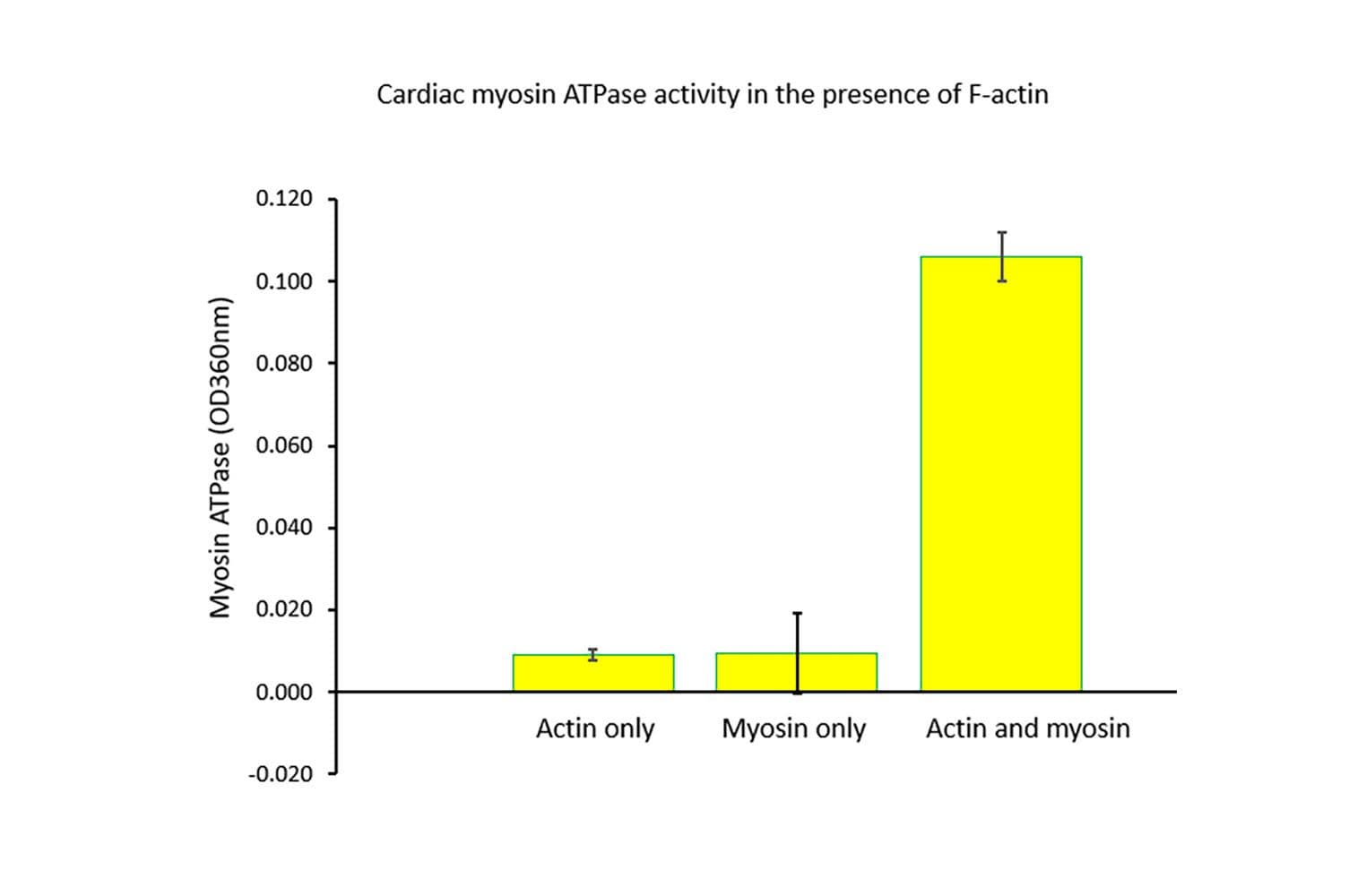
The biological activity of cardiac myosin is measured by its rate of F-actin–activated ATP hydrolysis. This is assessed using a standard in vitro F-actin ATPase assay. Quality control requires that, in the presence of F-actin, the ATP hydrolysis rate must be at least 10 times greater than in its absence, and the actin-activated ATPase activity must exceed 25 nmol/min/mg of myosin under the experimental conditions (see datasheet).
Cat. #MY03

© 2026 Cytoskeleton, Inc All Rights Reserved.