+3
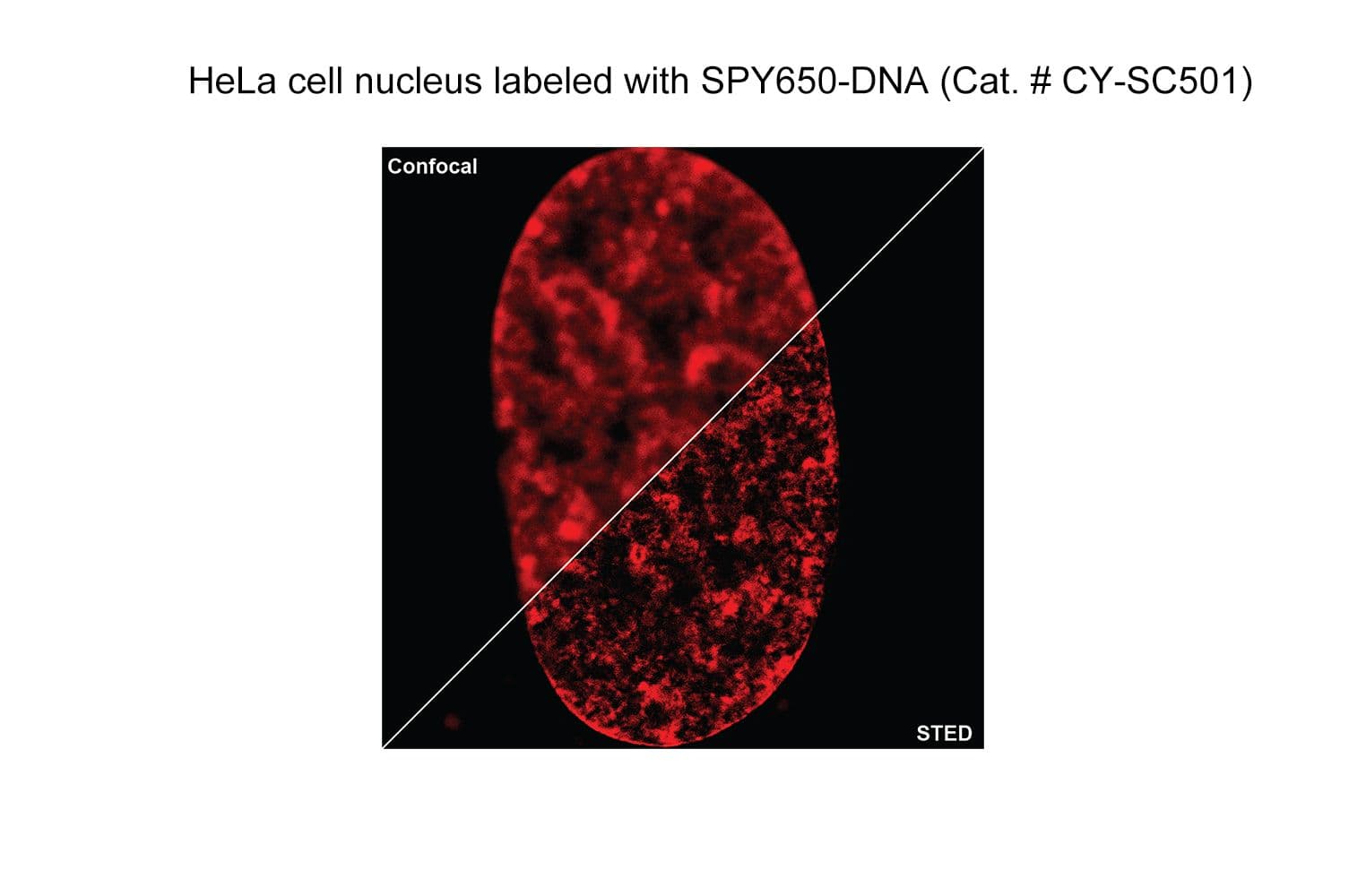
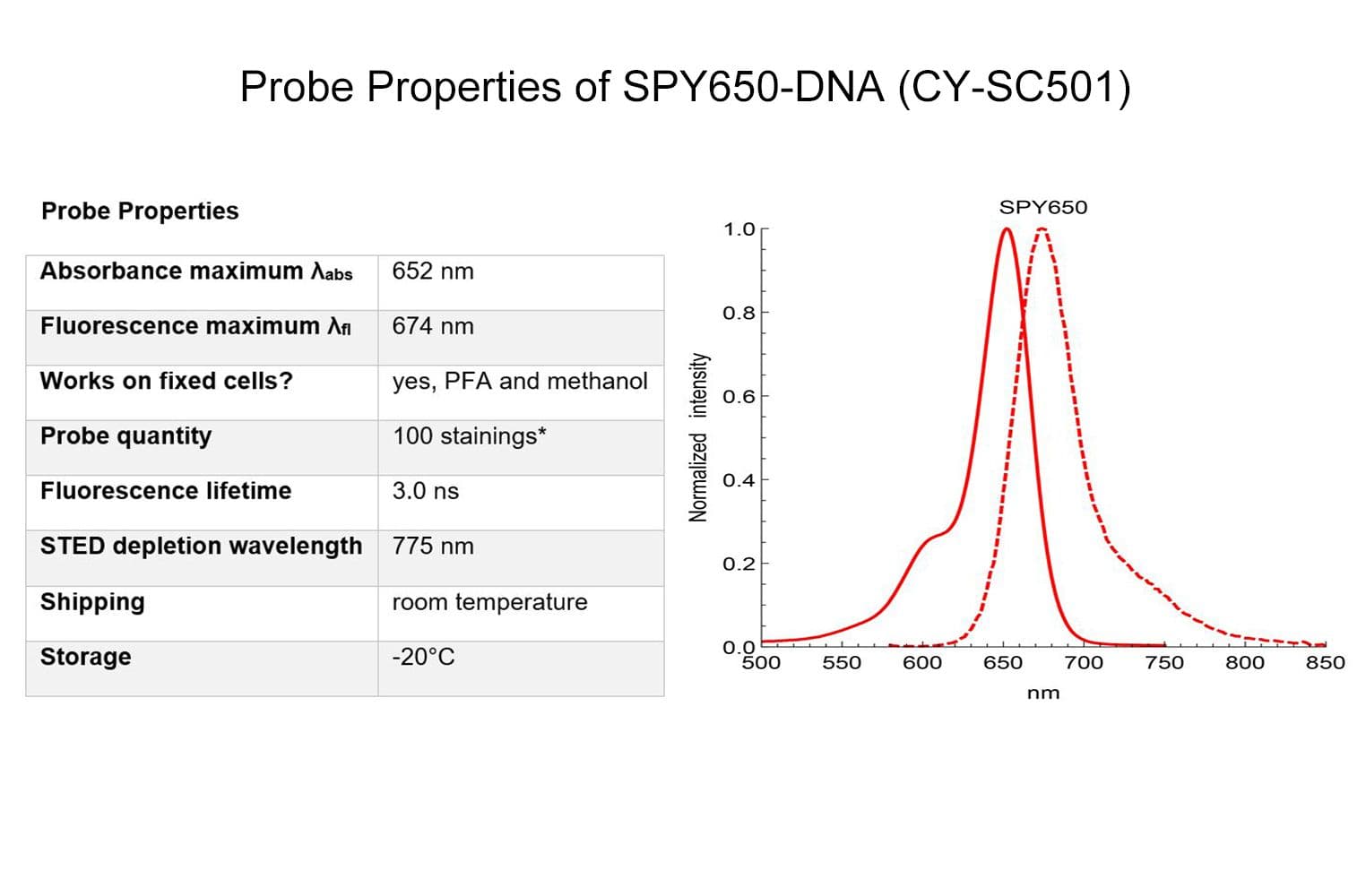
SPY650-DNA reagent is a conjugate of SPY™ dye and the DNA minor groove binder bisbenzimide (Hoechst). It allows the labelling of DNA in live cells with high specificity and low background.
Key features
The biological activity of CY-SC501 is assessed by the ability of the probe to efficiently label DNA/nuclei in live HeLa cell culture. Imaging performance has determined this probe to be less toxic and to have superior brightness, stability, and live cell permeability than the SiR-DNA probe. After an optional wash step, cell staining is visible for several hours. Omit the wash step for time-lapse imaging.