Pre-formed, high-purity actin filaments—ready-to-use for myosin motor assays, drug screening, and actin-binding studies. Skip the prep and get straight to reliable results.
Pre-formed actin filaments (PAFs) are prepared from rabbit skeletal muscle actin protein with a purity greater than 99% (Cat. # AKL99). These filaments undergo rigorous quality control to ensure high reliability and reproducibility in assays that require actin filament substrates. The average filament length is 8 µm, providing consistent performance across experiments.
PAFs are supplied as a lyophilized powder for convenience and stability.
PAFs are prepared from AKL99 actin protein. AKL99 is >99% pure alpha-skeletal muscle actin.
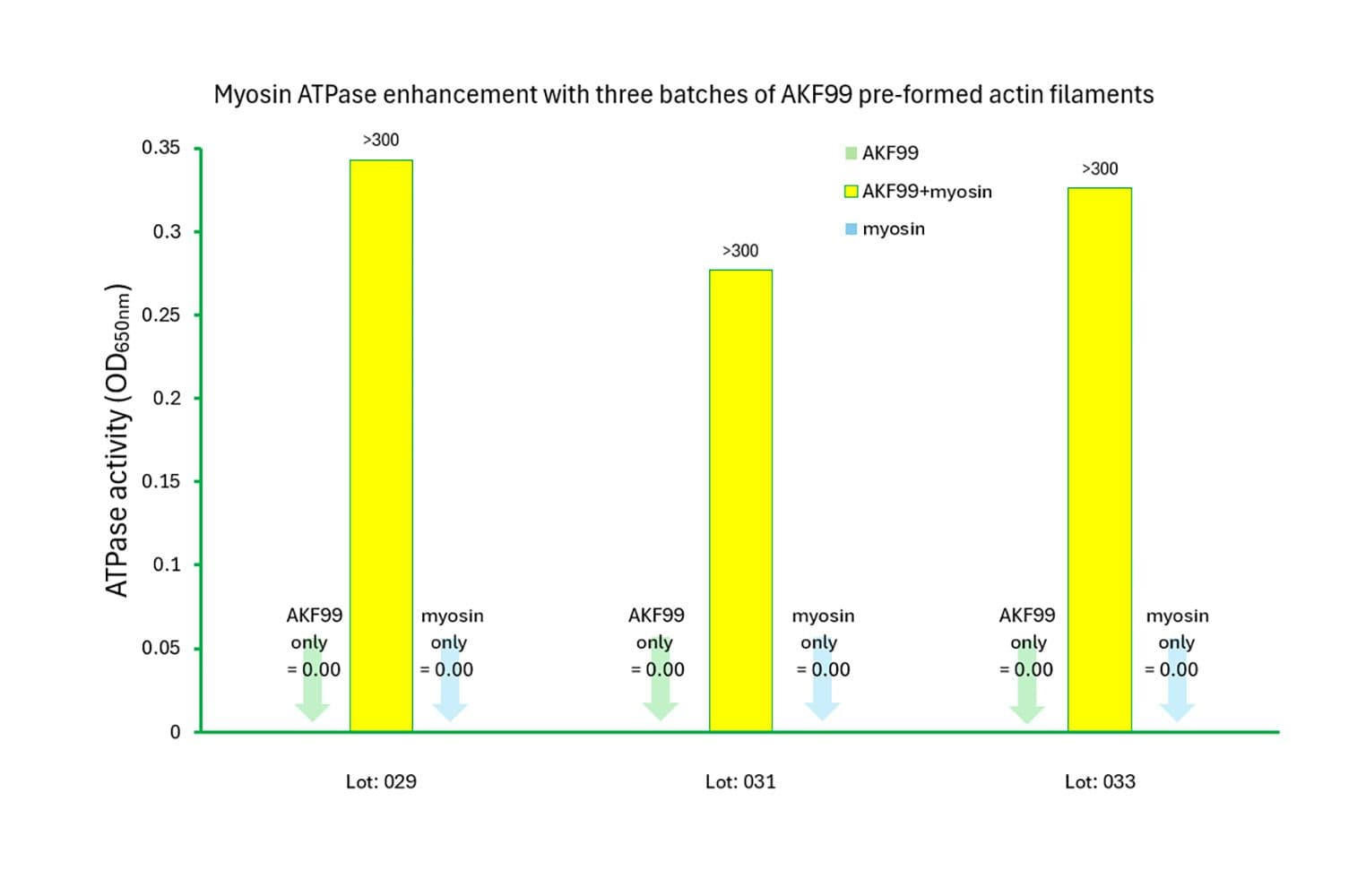
The biological activity of PAFs is assessed by analyzing polymer mass and their capacity to activate myosin ATPase. Stringent quality control measures ensure that over 85% of the actin is in filamentous form and that myosin ATPase activity is stimulated by at least tenfold compared to myosin or actin alone.
When used in actin filament–activated myosin ATPase assays, PAFs produce highly consistent results. The coefficient of variation (CV) for Vmax is less than 5% between samples and less than 8% across different batches.
Cat. #AKF99