+3
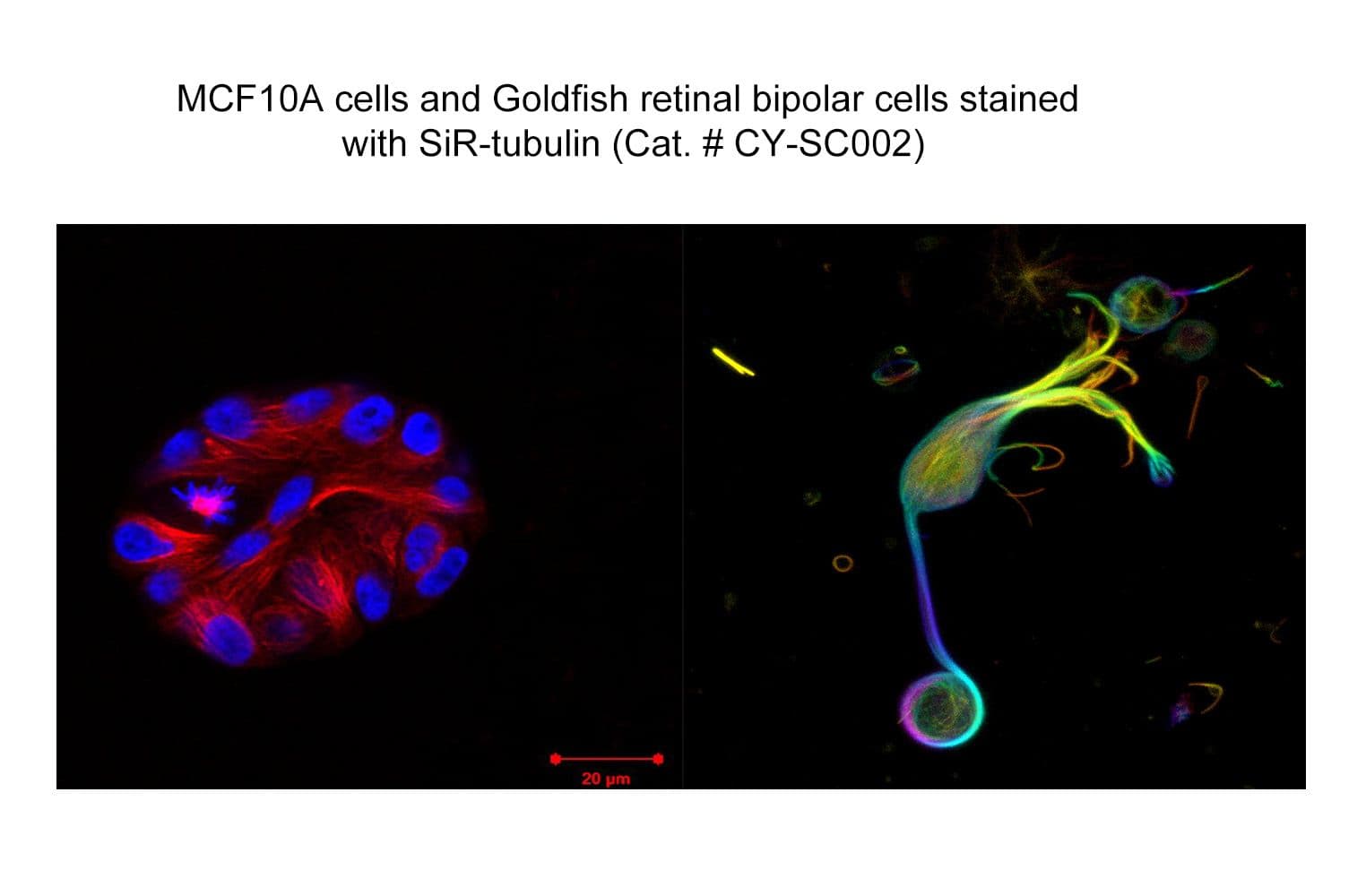
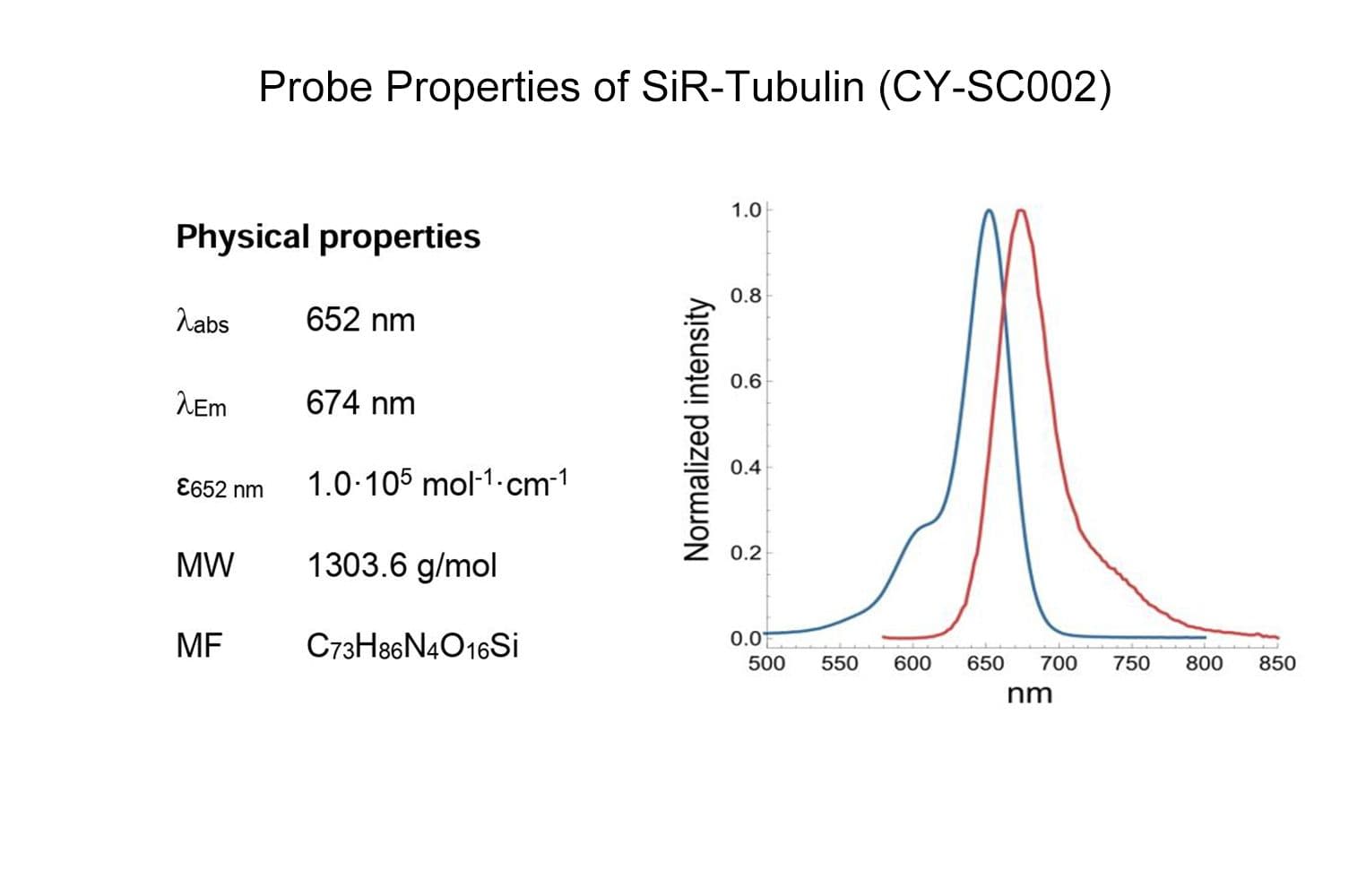
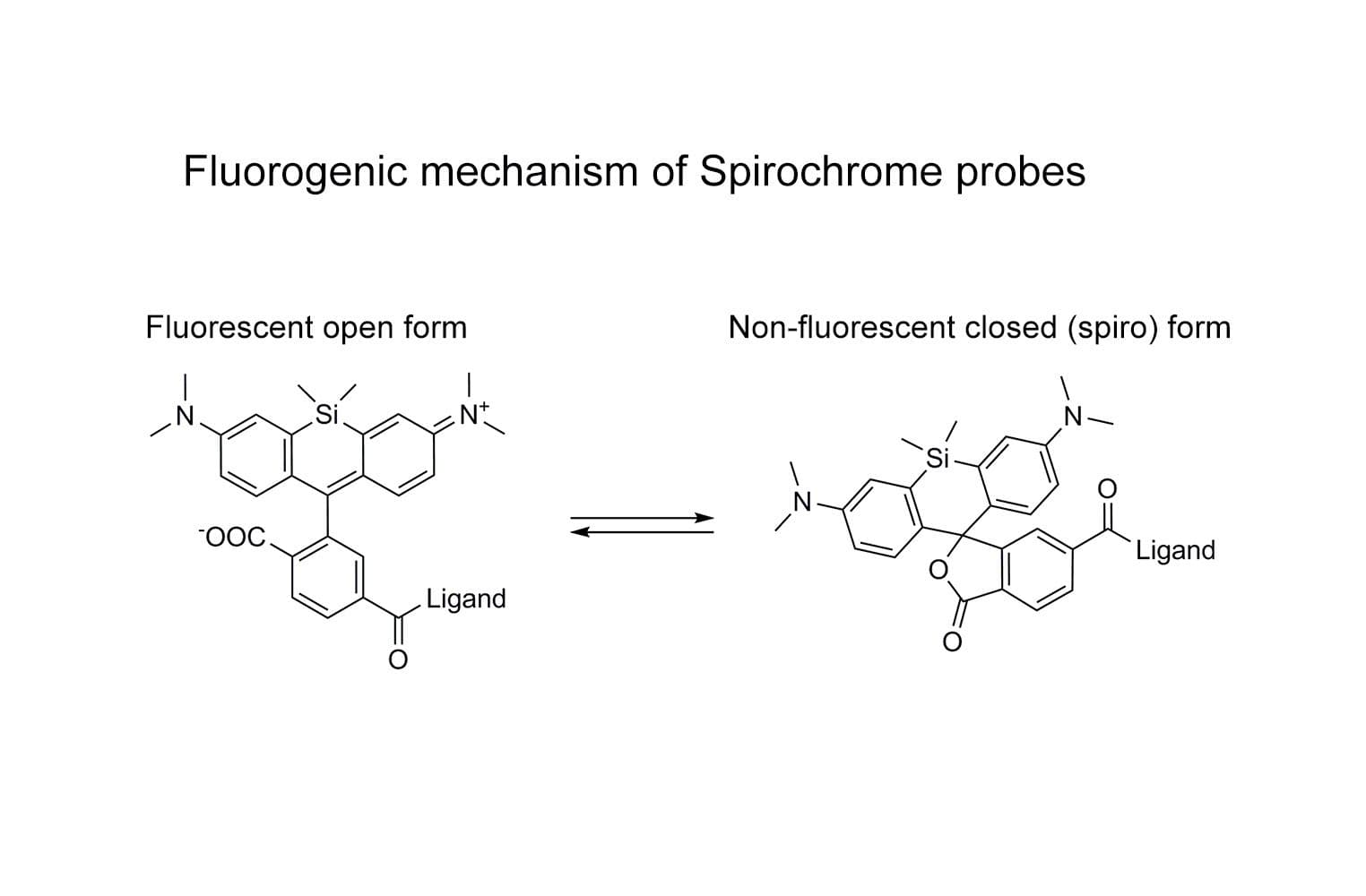
SiR-tubulin is based on the fluorophore silicon rhodamine (SiR) and the microtubule binding drug docetaxel. Sir-tubulin allows the labelling of microtubules in live cells with high specificity and low background.
Key features
The biological activity of CY-SC002 is assessed by the ability of the probe to efficiently label microtubules in live cell HeLa culture. After a wash step, cell staining is visible for several hours. SiR-tubulin is not recommended for fixed cells.
Cat. #CY-SC002