The acetylation of Lysine 40 (K40) found on the luminal side of MTs of a- tubulin is the most studied acetylated mark of tubulin. Abnormal acetylation has been linked to neurodegenerative disorders, ciliopathies, and cancers.
Acetylated tubulin was prepared by treating 99% pure porcine brain tubulin T240 with -tubulin N-acetyltransferase 1 (αTAT1) CS-TAT01 protein. The αTAT1 enzyme specifically modifies α-tubulin at lysine 40.
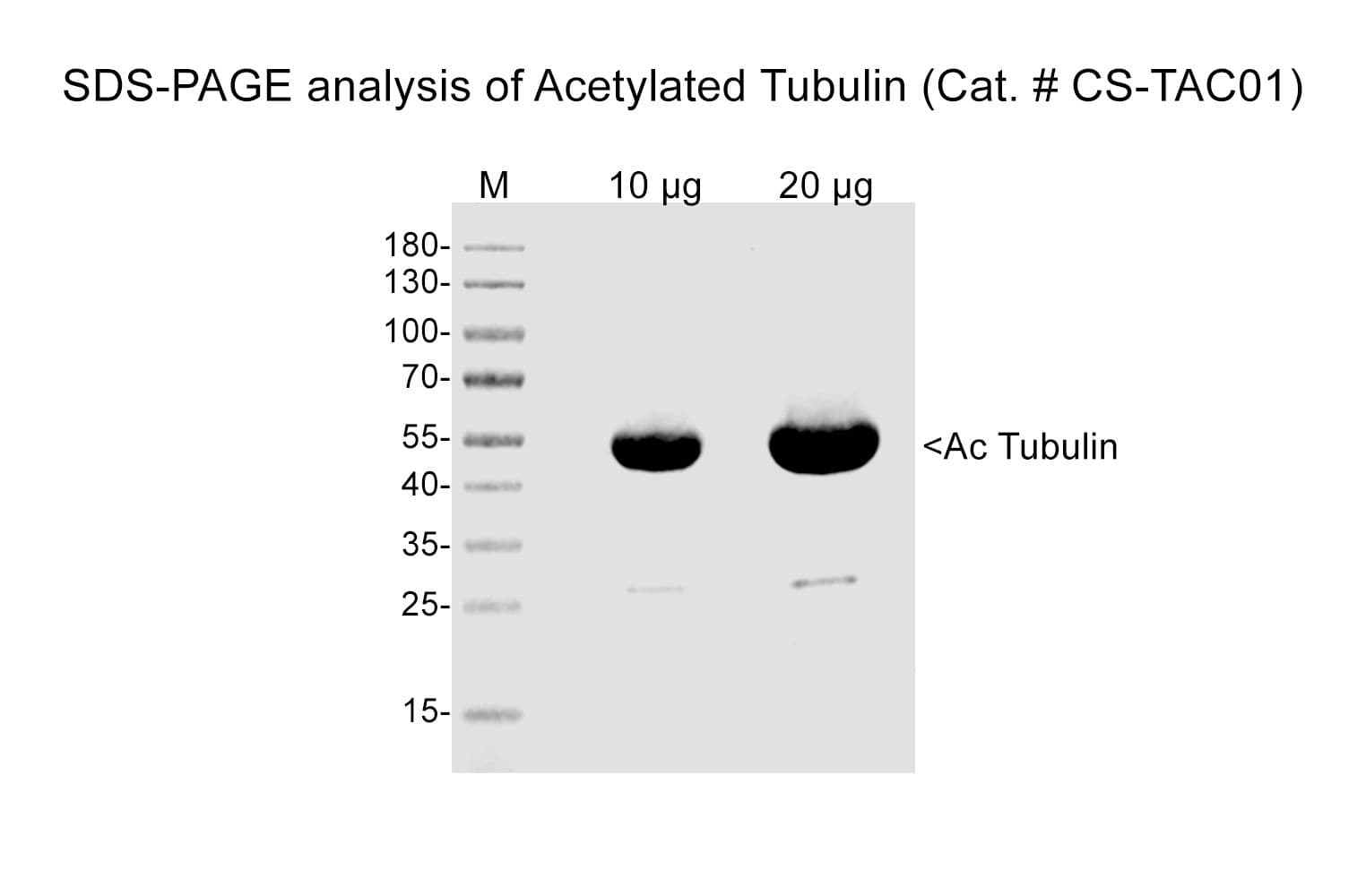
Protein purity is assessed by scanning densitometry of Coomassie Blue stained protein on a12% polyacrylamide gel. Purity is determined to be >99% pure.
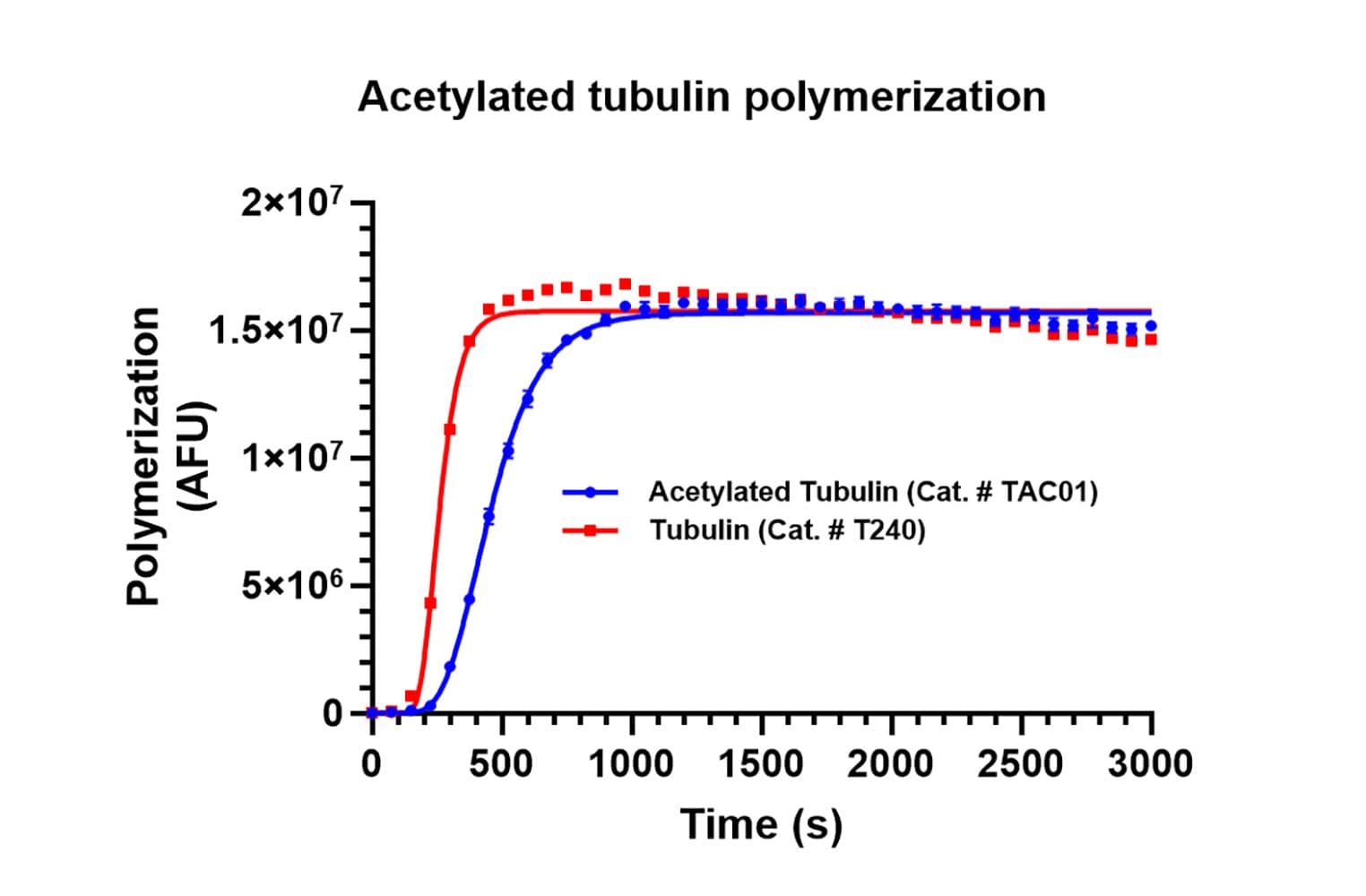
Tubulin acetylation was confirmed by an acetyl-lysine immunoprecipitation (IP) assay in which tubulin monomer was IP’d and quantitated. Quantitation of total acetylated tubulin in the IP indicated ≥90% of the α-tubulin was modified. Biological activity of acetylated tubulin was confirmed using a tubulin polymerization assay. Polymerization was found to reach a polymer mass equivalent to unmodified tubulin, acetylated tubulin displayed slower polymerization kinetics.
Cat. #CS-TAC01