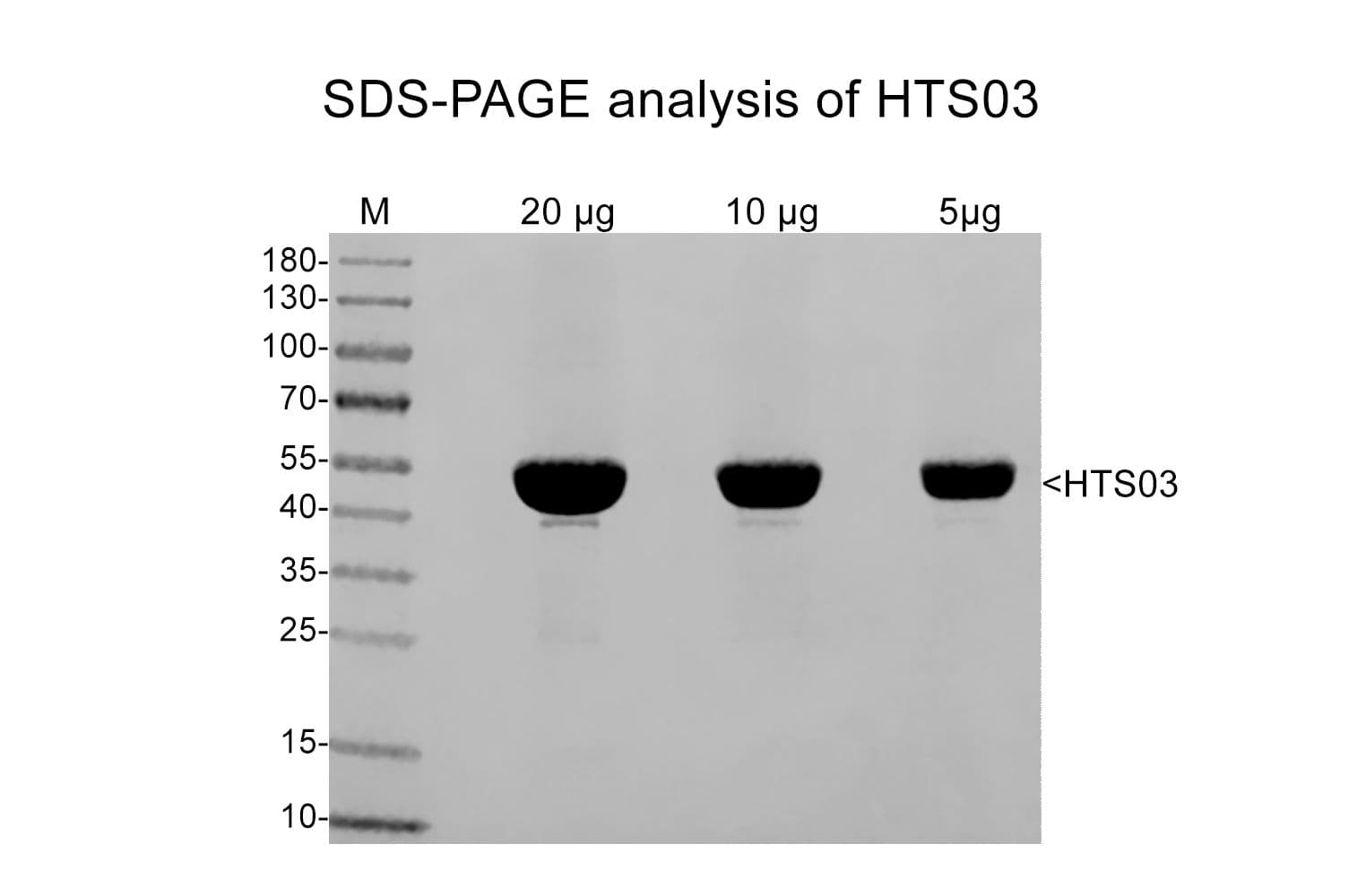
+3
Kit content (sufficient for 24 assays)
Equipment & materials required
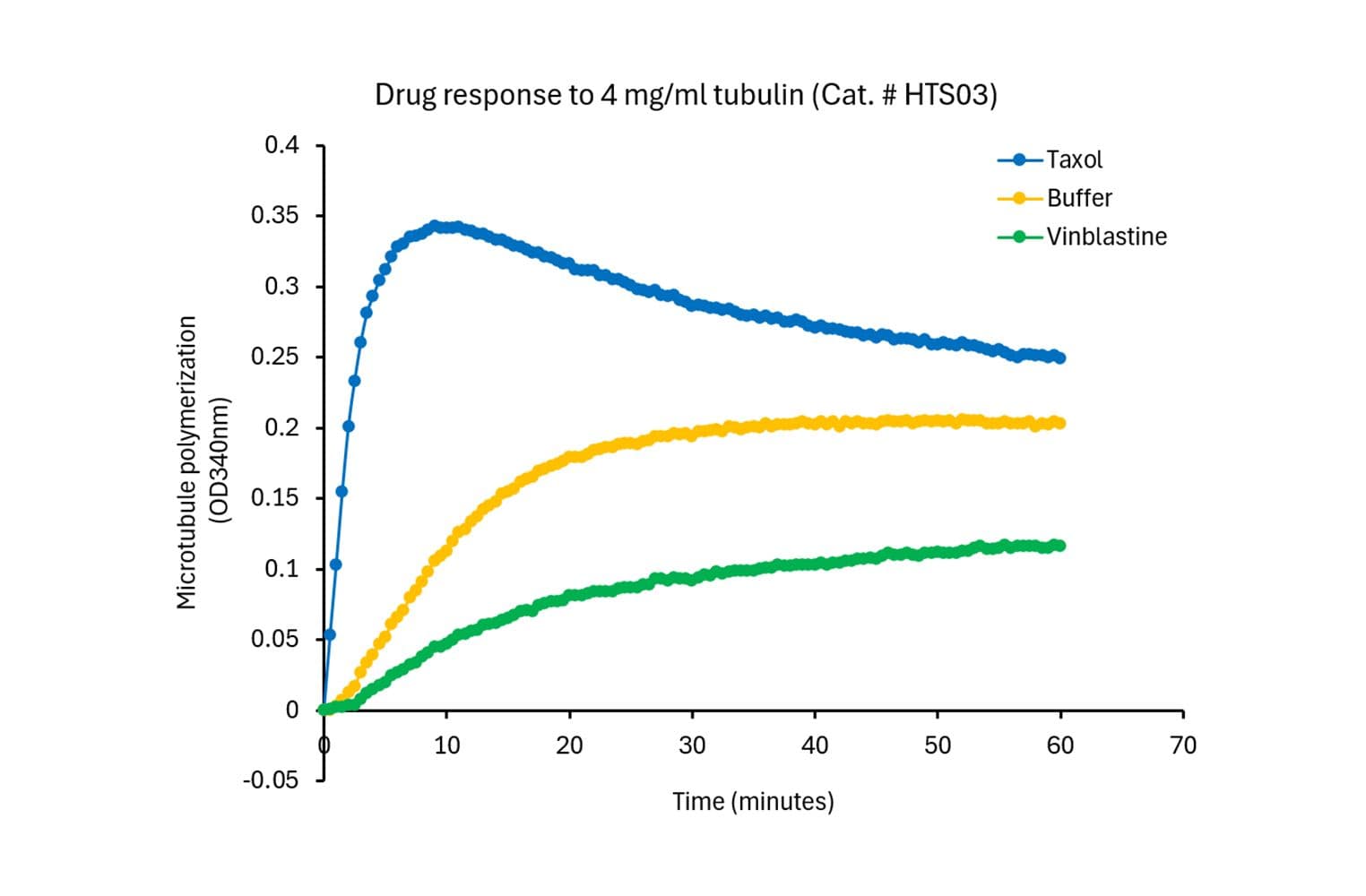
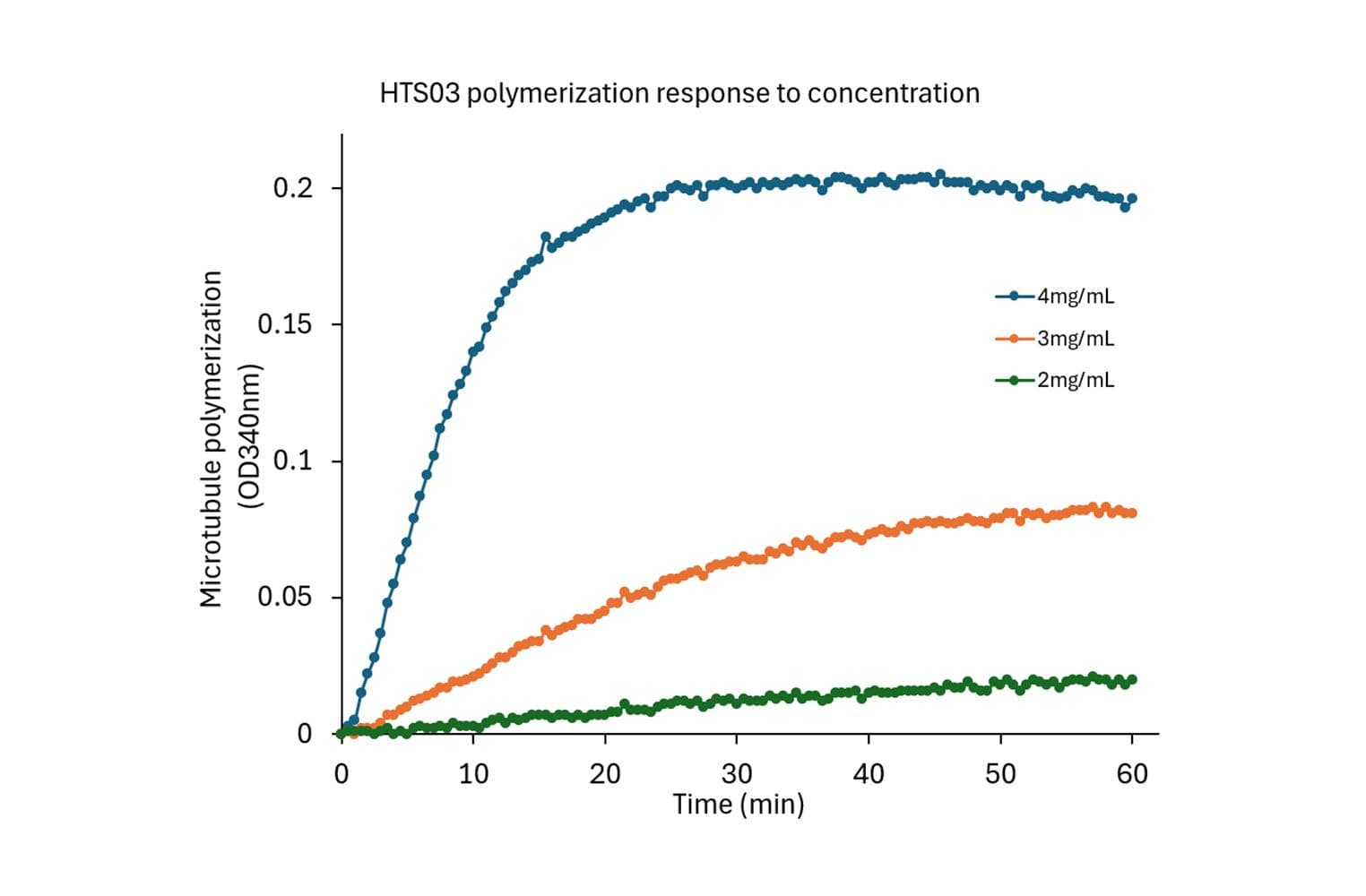
This kinetic assay measures tubulin polymerization dynamics by measuring the increase in light scattering (absorbance) as tubulin polymerizes into microtubules. It is designed to monitor the real-time kinetics of microtubule assembly and disassembly, supporting studies of cellular processes and drug screening.
Key features
Cat. #BK004P