+3
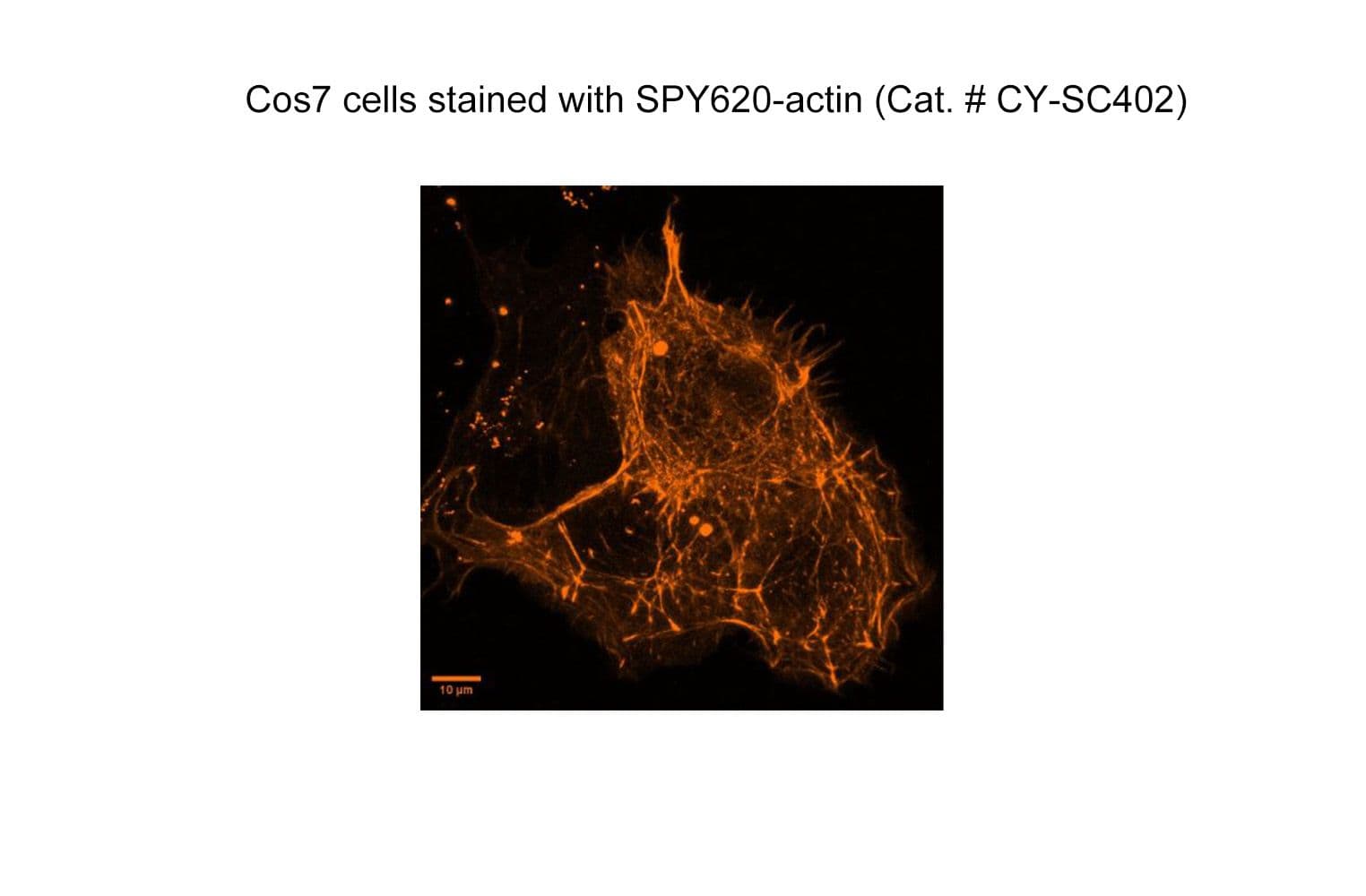
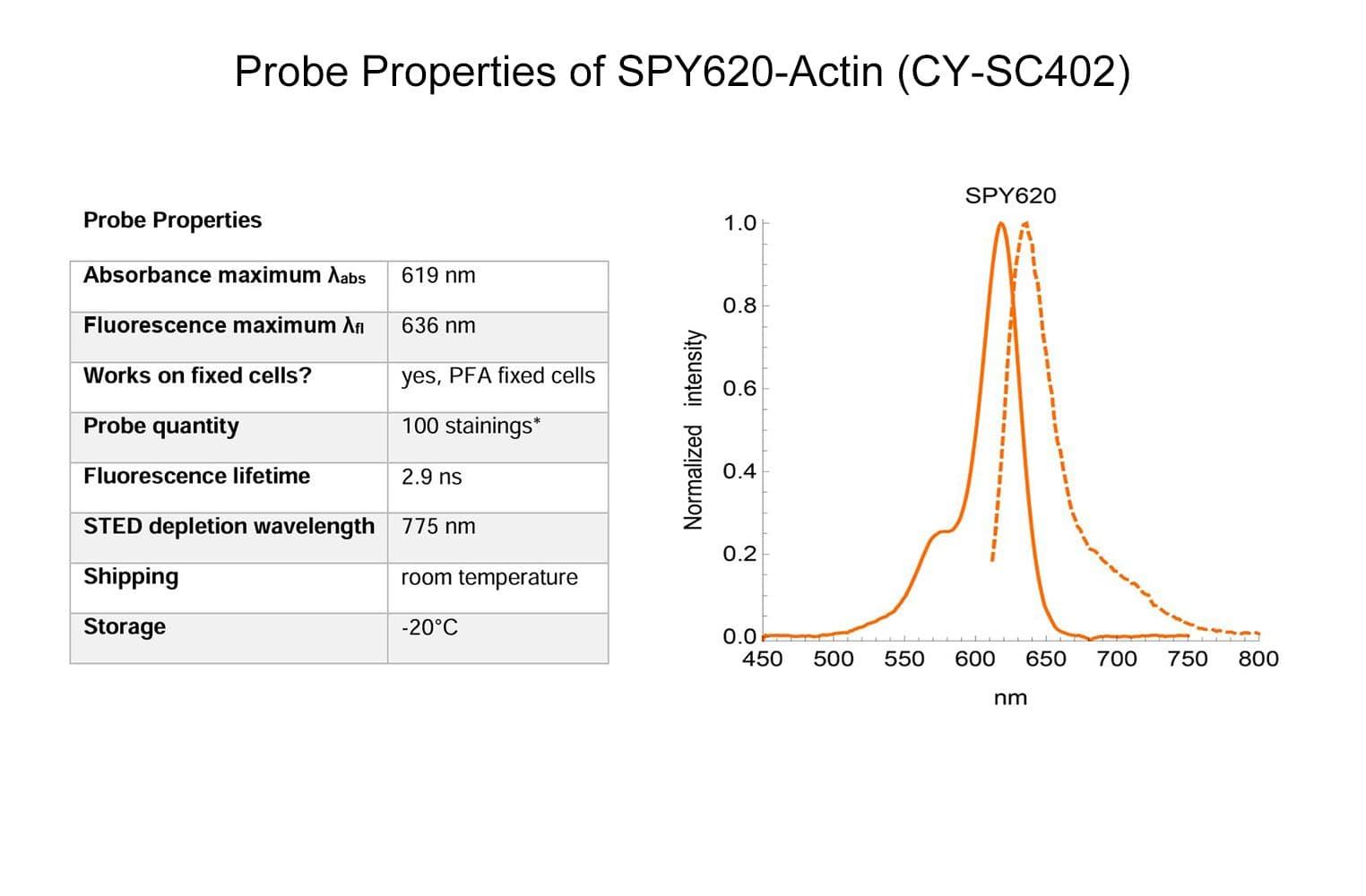
SPY™620-actin probe comprises a SPY™ dye conjugated to a derivative of the actin binding natural product jasplakinolide. Its fluorogenic nature allows the labelling of F-actin in live and fixed cells with high specificity and low background.
Key features
The biological activity of CY-SC402 is assessed by the ability of the probe to efficiently label actin filaments in live HeLa cell culture. After a wash step, cell staining is visible for several hours. If performing longer term time-laps readings it is recommended to keep the probe in the image media with no wash step.
Cat. #CY-SC402

© 2026 Cytoskeleton, Inc All Rights Reserved.