Gelsolin belongs to a class of actin-severing and capping proteins called class I F-actin capping proteins. Each of these class I proteins contains a series of conserved 125-150 amino acid repeat motifs. Gelsolin is characterized by the presence of six repeated motifs, three of which are actin-binding domains. Gelsolin has a regulatory role on actin filament length and its activity can be modulated by Ca2+ levels, pH, polyphosphoinositides, and post-translational modification. Plasma gelsolin differs from cytoplasmic gelsolin in that it is secreted from the cells and contains a 25-amino acid N-terminal extension.
The human plasma gelsolin has been produced in a bacterial expression system.
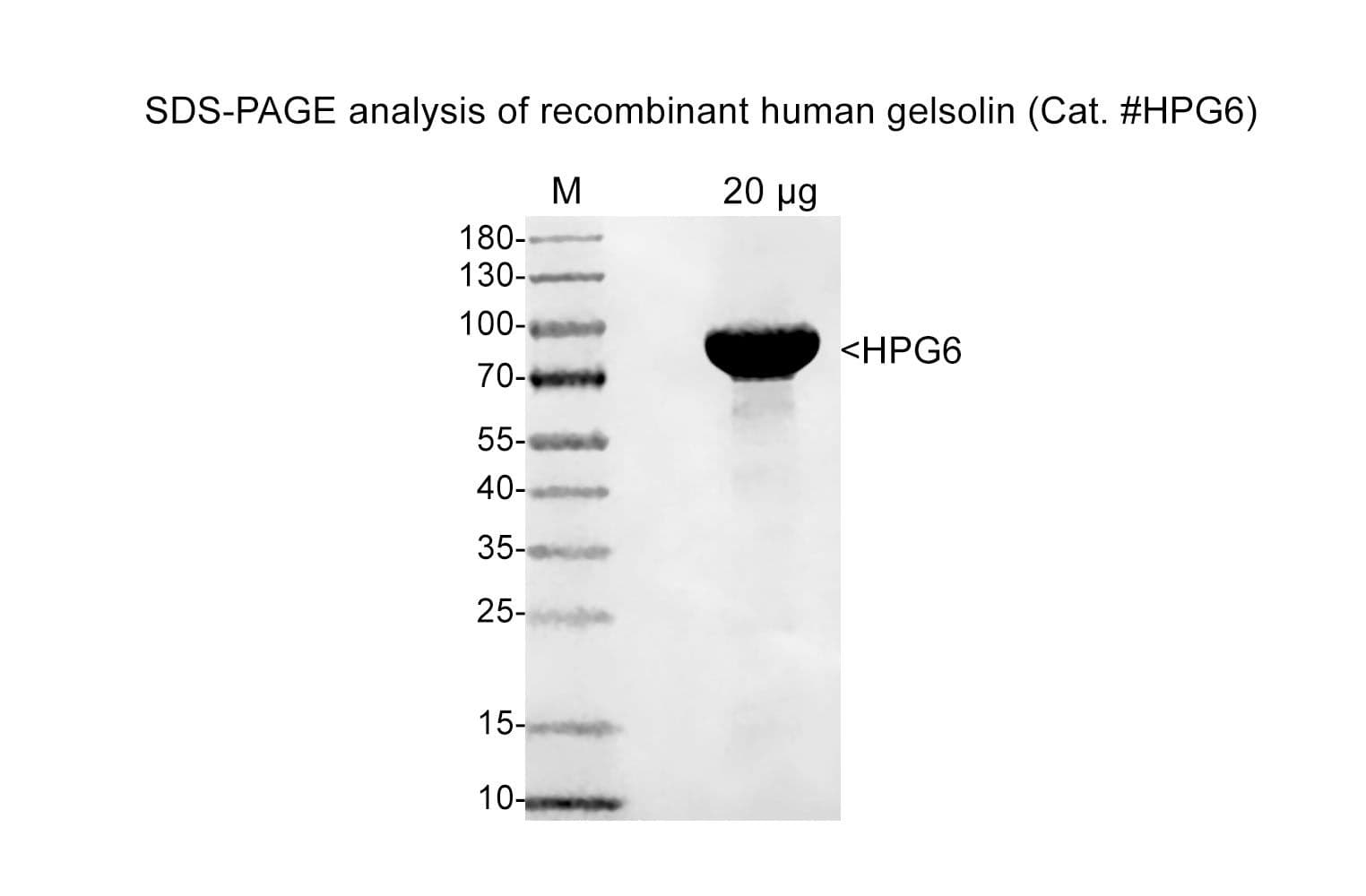
Protein purity is determined by scanning densitometry of Coomassie Blue-stained protein on a 4-20% polyacrylamide gel. Purity is ≥85%
The biological activity of recombinant gelsolin is assessed by its ability to sever F-actin as detected by an actin pelleting assay (see datasheet for details). In the absence of gelsolin,> 75% of F-actin is found in the pellet fraction. Upon incubation with gelsolin, >70% of the F-actin is found in the soluble G-actin form in the supernatant fraction.
Quality control ensures these severing patterns are consistently observed by SDS-PAGE analysis.
Cat. #HPG6