The Ras homolog gene family, member A, (RhoA) is one of three isoforms of the Rho sub-family of GTPases, part of the larger Ras super-family of GTPases, and the best characterized and most studied member of the sub-family. Full-length RhoA protein consists of 193 amino acids and has an approximate molecular weight of 22 kDa. RhoA is made up of an effector domain, four separate guanosine phosphate binding regions, a hypervariable region (residues 173-189), and a CAAX box motif (C: Cys; A: aliphatic residue; X: any residue). The effector domain (residues 26-45) changes conformation between the GTP-bound (active) and GDP-bound (inactive) states. Within the effector domain is the switch 1 (residues 27-40) region, which in conjunction with the switch 2 (residues 59-78) region change conformation between GTP- and GDP-bound states and are involved in the binding of downstream effectors. RhoA protein is targeted by several bacterial toxins, which modify key conserved amino acids, modifying RhoA’s functional activity. Toxins include Clostridium botulinum exoenzyme C3 transferase, which modifies Asn41, and Toxin B, which acts on Thr37. The hypervariable regions may contain sites for palmitoylation and a polybasic region which can determine membrane association. The C-terminus of RhoA is essential for correct localization of the protein. RhoA is post-translationally modified by prenylation of a conserved C-terminal cysteine followed by methylation and proteolytic removal of the last three amino acids. The prenyl group (geranylgeranyl) anchors the GTPase to membranes and this modification is essential for its stability, cell growth, transformation, and cytoskeletal organization. RhoA localizes predominantly to the plasma membrane and cytoplasm and is ubiquitously expressed. It also localizes to cell-cell contacts and cell projections.
RhoA GTPase behaves as a molecular switch in the regulation of multiple cellular processes. RhoA cycles between an inactive (GDP-bound) and active (GTP-bound) state. Guanine nucleotide-exchange factors (GEFs) activate Rho GTPases by catalyzing the exchange of GDP for GTP, and GTPase-activating proteins (GAPs) inactivate GTP-bound RhoA by enhancing intrinsic GTP hydrolysis activity. RhoA can also be maintained in an inactive state through sequestration in the cytoplasm by guanine nucleotide-dissociation inhibitors (GDIs), which bind prenylated GDP-bound Rho proteins, allowing translocation of Rho GTPases between membranes and cytosol.
RhoA regulates the dynamic organization of the actin cytoskeleton (e.g., polymerization, actomyosin contractility, stress fiber formation) and the cellular functions dependent on the dynamic actin cytoskeleton which includes cell morphology, polarity, cell adhesion, cell migration/motility, cell protrusion, exocytosis, endocytosis, cell cycle regulation, cytokinesis, development of the cell and its processes (ie., neurites), transcriptional control, and cell proliferation. RhoA is also involved in the regulation of microtubule dynamics. RhoA is believed to act primarily at the rear of migrating cells to promote detachment while its activity in the front of cells is dynamic to allow the extension and retraction of filopodia and lamellipodia. RhoA also plays a key role in regulating the integrity of cell-extracellular matrices and cell-cell adhesions, the latter including both adherens junctions and tight junctions. Loss of cell-cell junctions is required for the migration of epithelial cells and may be regulated reciprocally by downstream Rho effector proteins. Also, RhoA is localized to developing neuronal axons and growth cones. Local RhoA translation regulates the neuronal cytoskeleton.
Changes in expression and/or activity of RhoA have been reported in a variety of cancers, including, but not limited to breast, ovarian, testicular, colorectal, bladder, prostate, and glioblastoma. In addition to cancer, RhoA is also implicated in diseases of the central nervous system, diabetic nephropathy, hypertension, and pulmonary hypertension.
RhoA Images
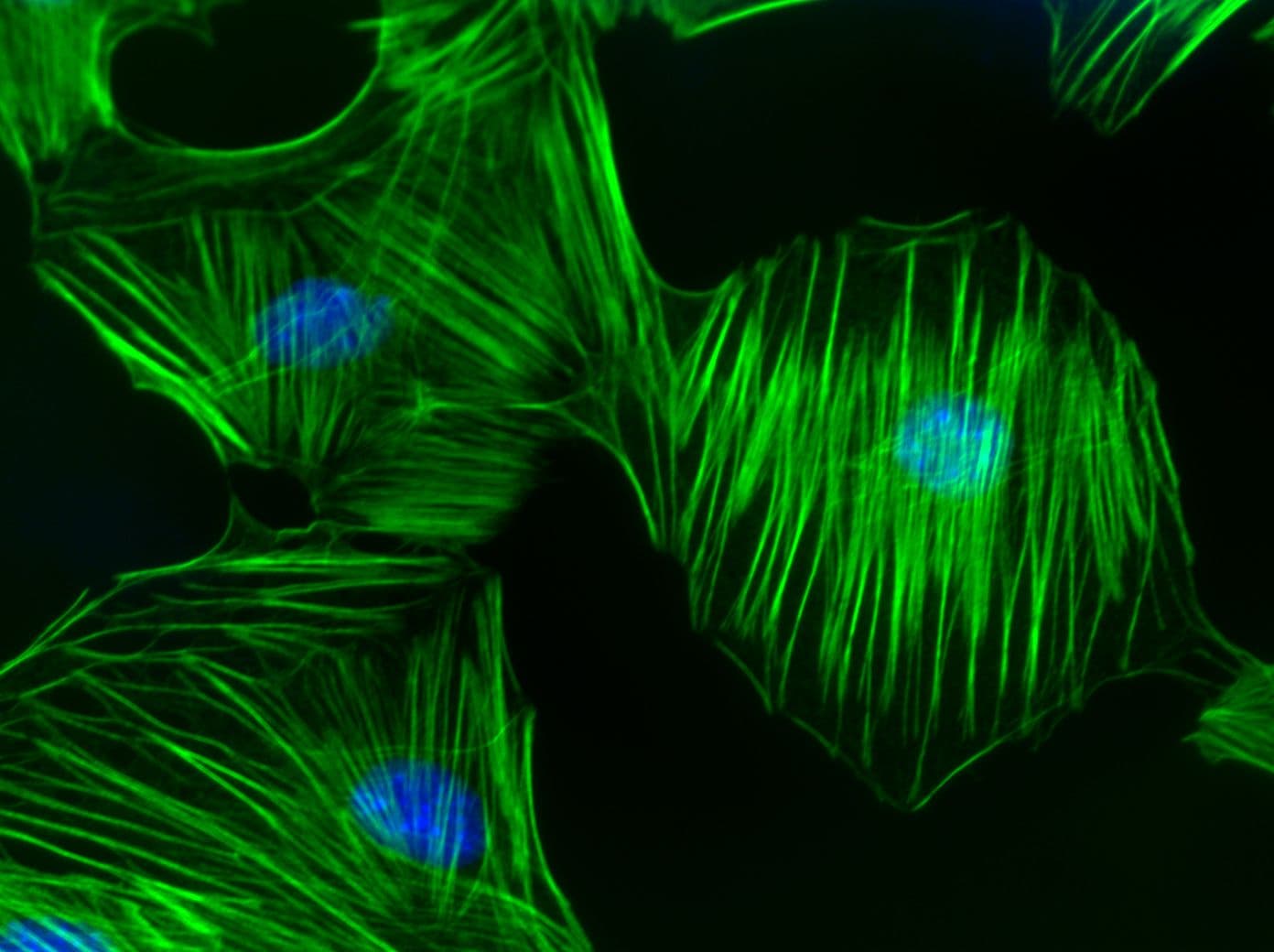
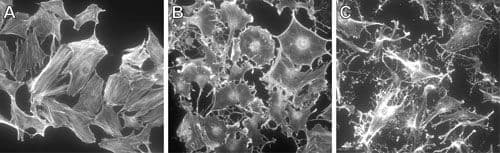
For more information about RhoA, please see:
Benitah S.A. et al. 2004. Rho GTPases in human cancer: an unresolved link to upstream and downstream transcriptional regulation. Biochim. Biophys. Acta. 1705, 121-132.
Bishop A.L. and Hall A. 2000. Rho GTPases and their effector proteins. Biochem. J. 348, 241-255.
Bos J.L. et al. 2007. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 129, 865-877.
Bustelo X.R. et al. 2007. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 29, 356-370.
Chi X. et al. 2013. Roles of rho GTPases in intracellular transport and cellular transformation. Int. J. Mol. Sci. 14, 7089-7108.
Etienne-Manneville S. and Hall A. 2002. Rho GTPases in cell biology. Nature. 420, 629-635.
Fortin Ensign S.P. et al. 2013. Implications of Rho GTPase signaling in glioma Cell invasion and tumor progression. Front. Oncol. 3, 241.
Fritz G. et al. 2002. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br. J. Cancer. 87, 635-644.
Fritz G. et al. 1999. Rho GTPases are over-expressed in human tumors. Int. J. Cancer. 81, 682-687.
Gomez del Pulgar T. et al. 2005. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays. 27, 602-613.
Gomez, del Pulgar T. and Lacal J.C. RHOA (ras homolog gene family, member A). Atlas Genet. Cytogenet. Oncol. Haematol. 11, 124-127.
Machacek M. et al. 2009. Coordination of Rho GTPase activities during cell protrusion. Nature. 461, 99-103.
Ridley A.J. et al. 2003. Cell migration: integrating signals from front to back. Science. 302, 1704-1709.
Rossman K.L. et al. 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167-180.
Wennerberg K. and Der C.J. 2004. Rho-family GTPases: it's not only Rac and Rho (and I like it). J. Cell Sci. 117, 1301-1312.
Wu K.Y. et al. 2005. Local translation of RhoA regulates growth cone collapse. Nature. 436, 1020-1024.
RhoA Related Products
Rho Activator II (Cat. # CN03)
Rho/Rac/Cdc42 Activator I(Cat. # CN04)
Acti-stain 488 phalloidin (Cat. # PHDG1-A)
Acti-stain 555 phalloidin (Cat. # PHDH1-A)
Acti-stain 670 phalloidin (Cat. # PHDN1-A)
Rhodamine Phalloidin (Cat. # PHDR1)
Anti-RhoA: mouse IgM Mab (Cat. # ARH05)
SiR-Actin Kit 50 nmol SiR-Actin and 1 umol verapamil (Cat. # CY-SC001)
RhoA Pull-down Activation Assay Biochem Kit (bead pull-down format) - 80 Assays (Cat. # BK036)
RhoA Pull-down Activation Assay Biochem Kit (bead pull-down format) - 80 Assays (Cat. # BK036-S)
RhoA G-LISA Activation Assay (Luminescence format) 96 assays (Cat. # BK121)
RhoA G-LISA Activation Assay Kit (Colorimetric format) 96 assays (Cat. # BK124)
RhoA / Rac1 / Cdc42 G-LISA Activation Assay Bundle 3 Kits (24 assays per kit) (Cat. # BK135)