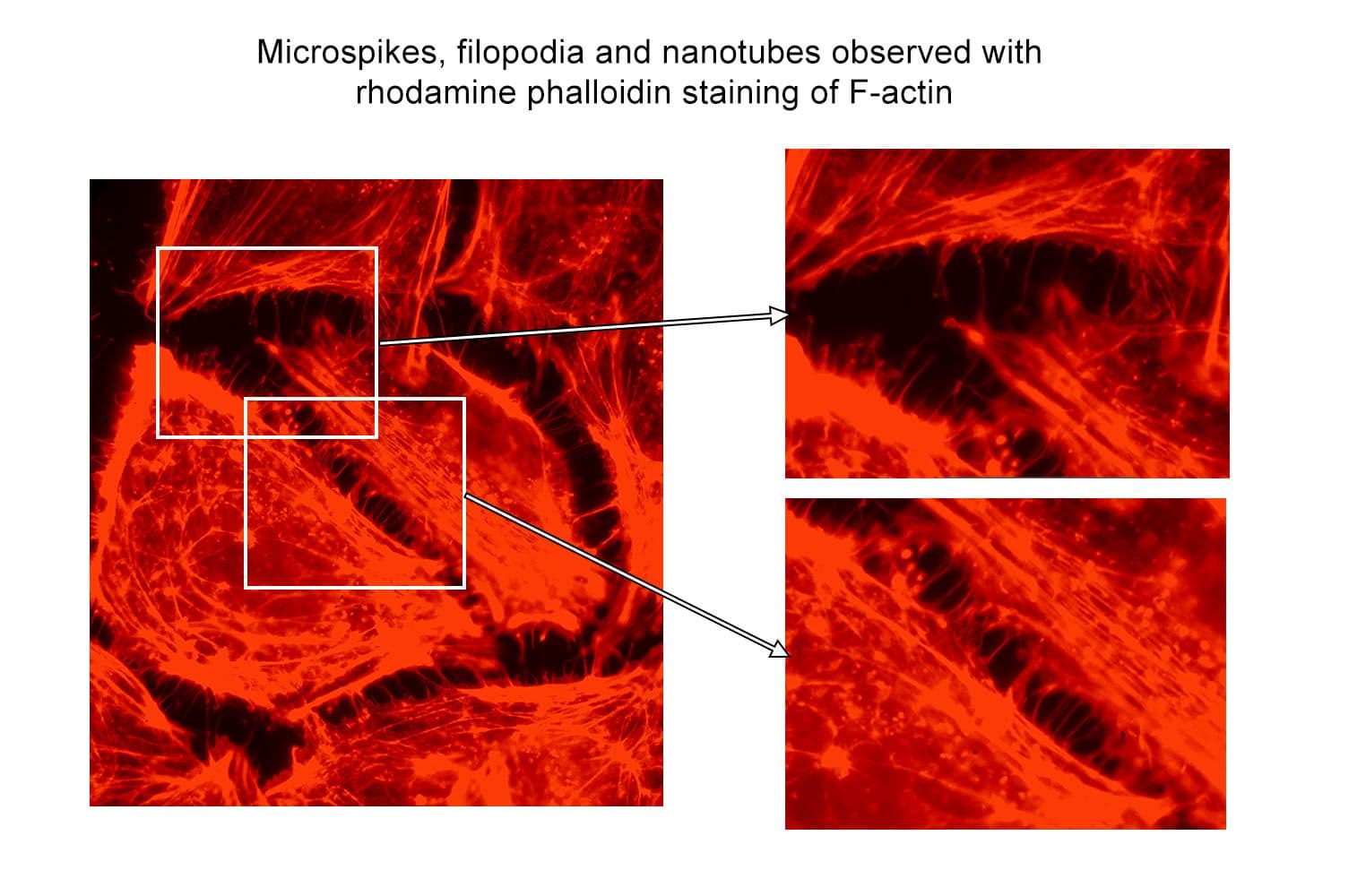
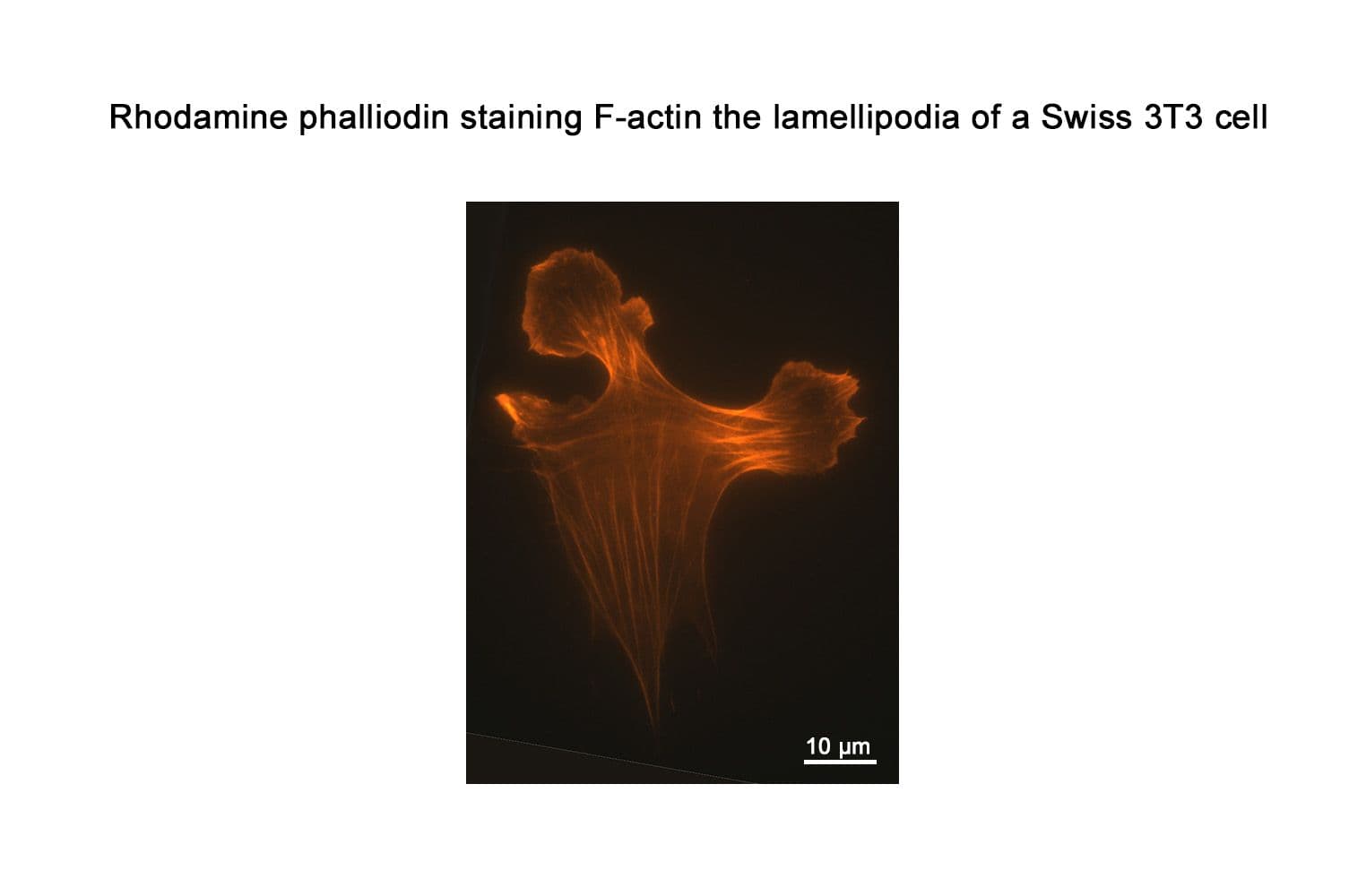
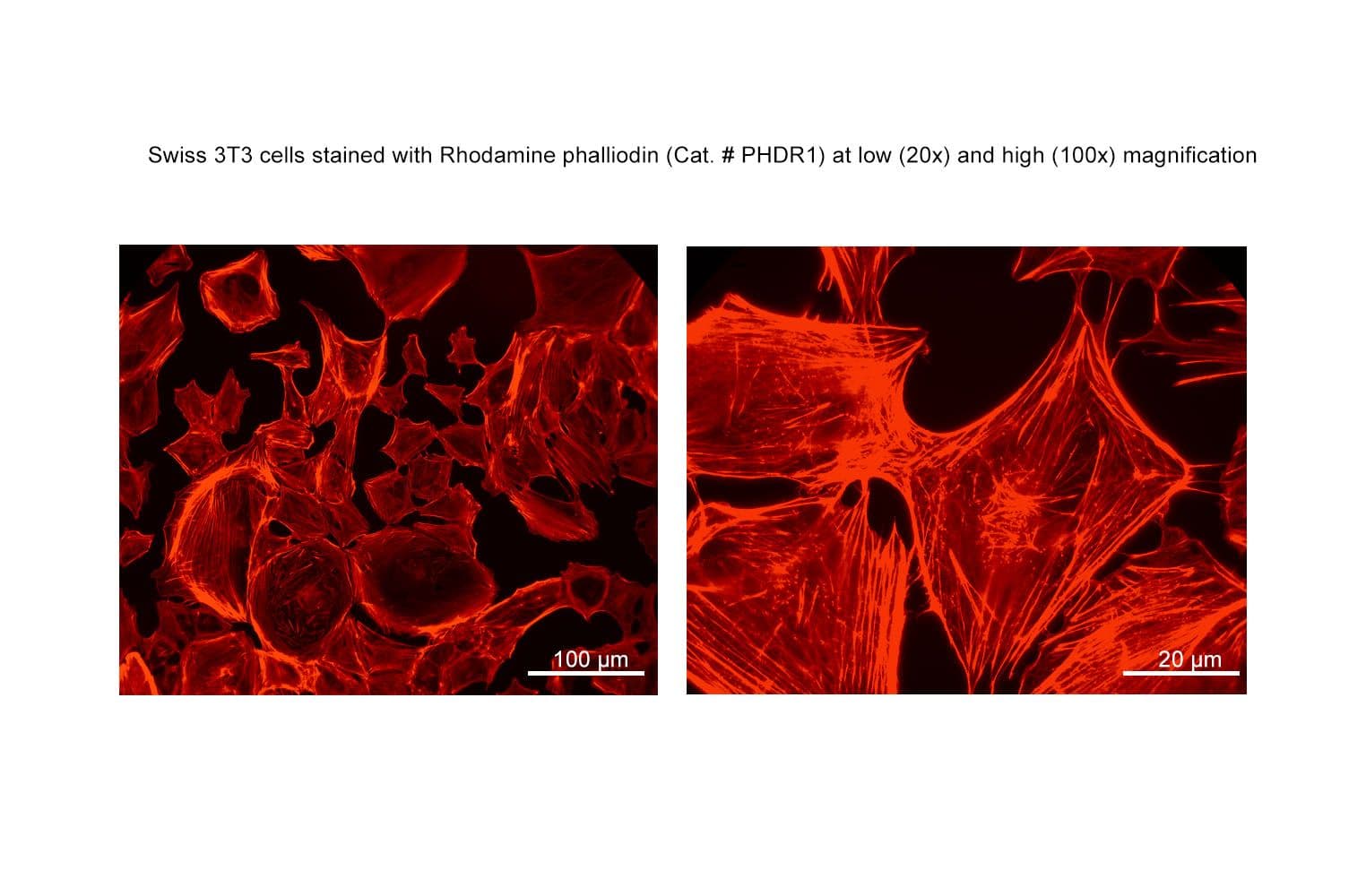
+3
Acti-stain™ rhodamine fluorescent phalloidin is a high-affinity probe for F-actin, enabling precise visualization of the actin cytoskeleton in fixed cells and tissues. Conjugated to bright, photostable fluorophores, it delivers sharp, low-background staining ideal for fluorescence microscopy and quantitative imaging applications.
Key characteristics
≥90% by HPLC
Biological activity of Acti-stain™ rhodamine is demonstrated by the ability of phalloidin to specifically stain actin filaments in 3T3 cells.
Cat. #PHDR1