+3
Non-muscle actin has been purified from human platelets. The isotype composition is 85% β-actin and 15% γ-actin.
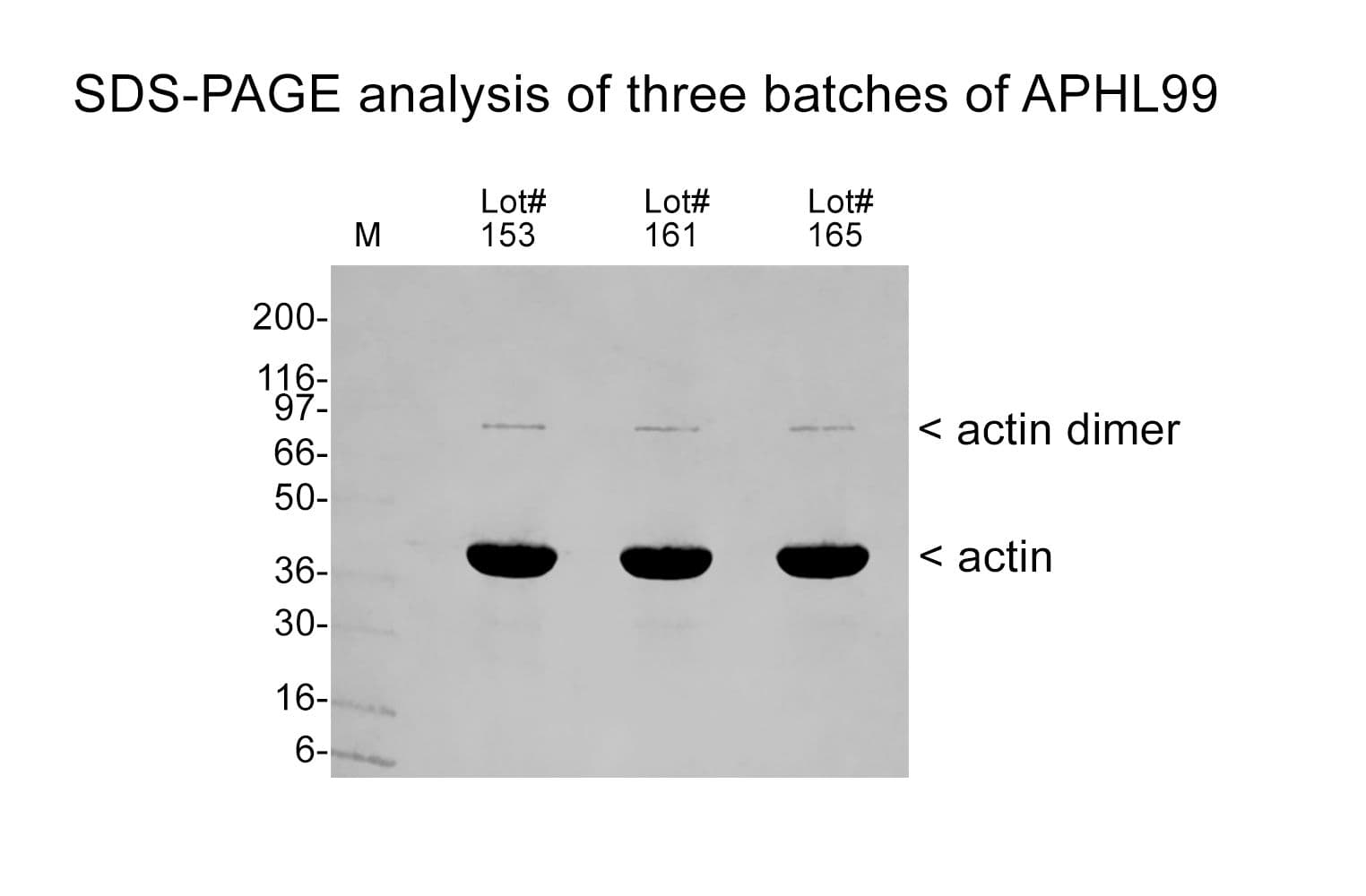
Non-muscle actin has an approximate molecular weight of 43 kDa. APHL99 is provided as a white lyophilized powder.
Protein purity is assessed using scanning densitometry of Coomassie-stained SDS-PAGE gels. APHL99 is >99% pure alpha-skeletal muscle actin.
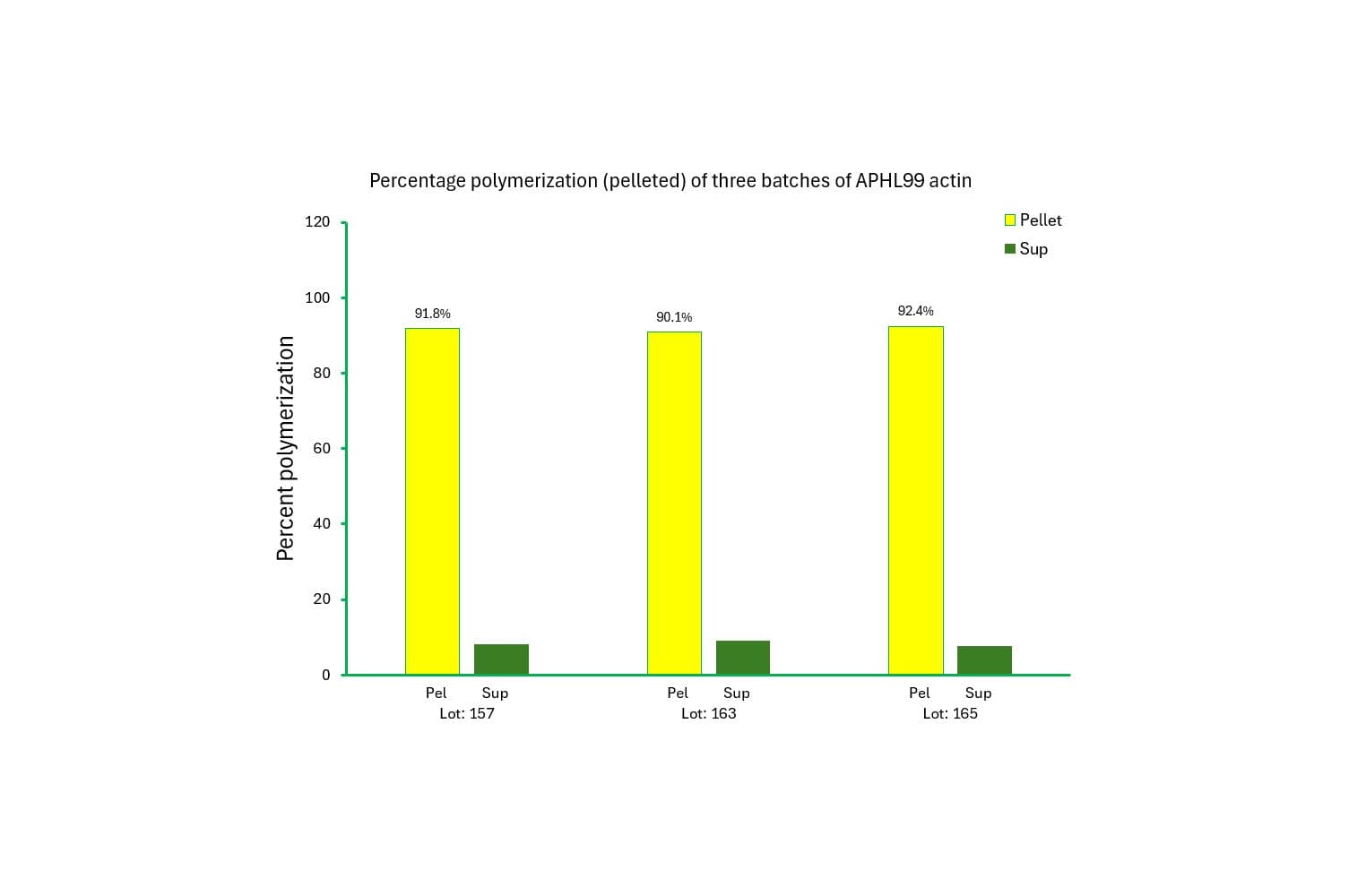
The biological activity of non-muscle actin is assessed by its ability to efficiently polymerize into filaments in vitro, which can be separated from unpolymerized components using a spin-down assay. Rigorous quality control ensures that >85% of the non-muscle actin polymerizes under these conditions.
Cat. #APHL99