Ras homolog gene family, member B (RhoB) is one of the three isoforms of the Rho family of GTPases, which is a sub-family of the larger Ras super-family of GTPases. The RhoB gene consists of 2,366 base pairs and 1 exon. The gene encodes a protein 196 amino acids long with a molecular weight of approximately 22 kDa. RhoB protein exists in different geranylgeranylated or farnesylated isoforms in cells. RhoB is the only Rho sub-family member that can be modified by palmitoylation. RhoB protein is targeted by the Clostridium botulinum toxin exoenzyme C3 transferase, which modifies amino acid Asn41, rendering the RhoB protein irreversibly inactive.
The RhoA, RhoB, and RhoC proteins form a closely related subgroup that are about 90% identical in amino acid sequences. The sequences of RhoB are highly-conserved between species (from human to fly). Amino acid sequences of human, mouse, and rat are 100% identical, while sequence homology between human and chicken is 97% identical. RhoB is localized to multiple intracellular locations, including endosomes, late endosomes, late endosomal membranes, cell membranes, the nuclear margin, and in the nucleus. Prenylation specifies the subcellular location of RhoB. In general, the farnesylated form is localized to the plasma membrane while the geranylgeranylated form is localized to the endosome.
Like the related isoforms RhoA and RhoC, RhoB plays a pivotal physiological role in the dynamic regulation of the actin cytoskeleton. RhoB is involved in intracellular protein trafficking of a number of proteins. For example, RhoB targets PRK1 (Serine/threonine-protein kinase N1; a.k.a. PKN) to endosomes and is involved in trafficking of the epidermal growth factor (EGF) receptor from late endosomes to lysosomes. RhoB is also required for stability and nuclear trafficking of Akt, which promotes endothelial cell survival during vascular development. RhoB has also been identified as a component of outside-in signaling pathways that coordinate Src activation with its translocation to transmembrane receptors.
In cancer biology, RhoB is regarded as a negative modulator of cancer progression (i.e., tumor suppressor) in its general inhibition of cell adhesion and growth factor signaling in the majority of transformed cells. Additionally, the loss of RhoB is correlated with increased Ras mutant-associated tumor formation and conversely, down-regulation of RhoB is associated with many different types of tumors. RhoB limits the proliferation of transformed cells through increased turnover of the c-Myc oncogene. Furthermore, RhoB promotes pro-apoptotic signaling of regulators involved in cell cycle checkpoints, cell adhesion, vesicle trafficking, mitogen-activated protein kinase (MAPK) signaling, transcription, and immunity. RhoB mediates apoptosis in neoplastically-transformed cells after DNA damage and is essential for apoptosis and anti-neoplastic activity of farnesyltransferase inhibitors in vivo. Thus, RhoB was/is one of the targets of farnesyltransferase inhibitors under study as cancer therapeutics.
RhoB Images
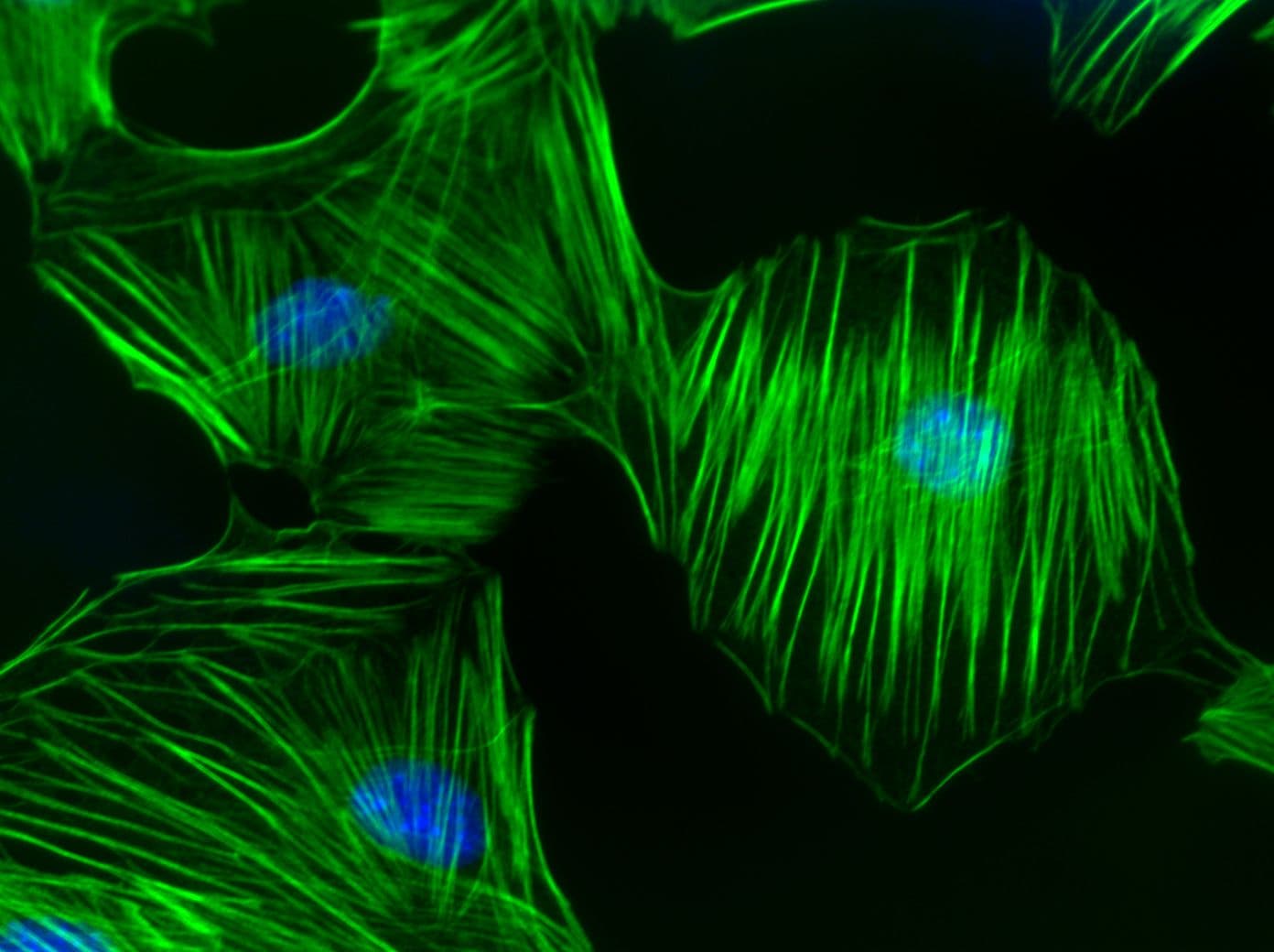
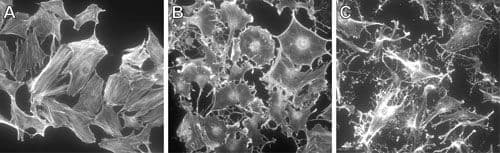
For more information on RhoB, please see here:
Benitah S.A. et al. 2004. Rho GTPases in human cancer: an unresolved link to upstream and downstream transcriptional regulation. Biochim. Biophys. Acta. 1705, 121-132.
Bishop A.L. and Hall A. 2000. Rho GTPases and their effector proteins. Biochem. J. 348, 241-255.
Bos J.L. et al. 2007. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 129, 865-877.
Bustelo X.R. et al. 2007. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 29, 356-370.
Chi X. et al. 2013. Roles of rho GTPases in intracellular transport and cellular transformation. Int. J. Mol. Sci. 14, 7089-7108.
Etienne-Manneville S. and Hall A. 2002. Rho GTPases in cell biology. Nature. 420, 629-635.
Fortin Ensign S.P. et al. 2013. Implications of Rho GTPase signaling in glioma Cell invasion and tumor progression. Front. Oncol. 3, 241.
Fritz G. et al. 2002. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br. J. Cancer. 87, 635-644.
Fritz G. et al. 1999. Rho GTPases are over-expressed in human tumors. Int. J. Cancer. 81, 682-687.
Gomez del Pulgar T. et al. 2005. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays. 27, 602-613.
Machacek M. et al. 2009. Coordination of Rho GTPase activities during cell protrusion. Nature. 461, 99-103.
Ridley A.J. et al. 2003. Cell migration: integrating signals from front to back. Science. 302, 1704-1709.
Rossman K.L. et al. 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167-180.
Wennerberg K. and Der C.J. 2004. Rho-family GTPases: it's not only Rac and Rho (and I like it). J. Cell Sci. 117, 1301-1312.
RhoB Related Products
Rho Activator II (Cat. # CN03)
Rho/Rac/Cdc42 Activator I(Cat. # CN04)
Acti-stain 488 phalloidin (Cat. # PHDG1-A)
Acti-stain 555 phalloidin (Cat. # PHDH1-A)
Acti-stain 670 phalloidin (Cat. # PHDN1-A)
Rhodamine Phalloidin (Cat. # PHDR1)
Anti-RhoA: mouse IgM Mab (Cat. # ARH05)
SiR-Actin Kit 50 nmol SiR-Actin and 1 umol verapamil (Cat. # CY-SC001)
RhoA Pull-down Activation Assay Biochem Kit (bead pull-down format) - 80 Assays (Cat. # BK036)
RhoA Pull-down Activation Assay Biochem Kit (bead pull-down format) - 80 Assays (Cat. # BK036-S)
RhoA G-LISA Activation Assay (Luminescence format) 96 assays (Cat. # BK121)
RhoA G-LISA Activation Assay Kit (Colorimetric format) 96 assays (Cat. # BK124)
RhoA / Rac1 / Cdc42 G-LISA Activation Assay Bundle 3 Kits (24 assays per kit) (Cat. # BK135)