KSP/Eg5 Inhibition in Cancer: Theory and Therapy
KSP (Eg5) Inhibition - Theory and Expreimental Studies
The Kinesin Spindle Protein (KSP; also known as Eg5 or KIF11) is a Kinesin-5 subfamily member and has been the focus of a significant drug development effort throughout the pharmaceutical industry for the last 15 years. KSP plays a critical role in mitotic spindle pole separation, and its inhibition results in the formation of monoaster spindles which is thought to lead to mitotic catastrophe and apoptosis1. From a therapeutic standpoint, it was anticipated that inhibiting KSP would produce antimitotic benefits by targeting microtubules with fewer and less severe side effects. Microtubule targeting drugs (e.g., Paclitaxel and Vincristine) are quite efficacious and continue to be a front line therapeutic approach for several cancers (e.g., ovarian, breast, gastroesophageal)2. These drugs, however, are associated with significant and occasionally debilitating side effects2,3. Although microtubules play a critical role in chromosomal segregation during mitosis, they also have important roles in postmitotic cells like neurons where vesicle transport can be disrupted by microtubule-targeted drugs, leading to peripheral neuropathies2,3. The original identification of the KSP inhibitor monastrol in 1999 by Mayer et al4 opened the door to a promising new direction for antimitotic drug discovery. Monastrol itself did not exhibit the appropriate drug-like characteristics necessary to be tested in human clinical trials; however, a growing list of second generation KSP inhibitors have been developed with improved pharmacokinetic properties.
Clinical Trial with KSP (Eg5) Inhibitors
In 2004, Ispinesib (SB-715992), developed by Cytokinetics and GlaxoSmithKline, became the first KSP inhibitor to enter clinical trials5. There have been 35 Phase 1/2 clinical trials initiated to date with 8 chemically distinct KSP inhibitors (see Table 1). The preponderance of evidence suggests that KSP inhibitors are well-tolerated and as predicted, they do not exhibit the neurotoxicities associated with microtubule-targeted drugs6. KSP inhibitors target rapidly dividing cells and consequently they do share dose-limiting toxicities with other antimitotic therapies, including neutropenia and leukopenia (lowered neutrophil and leukocyte levels, respectively)6. Unfortunately, many of these KSP inhibitors have failed to show efficacy in a clinical setting as a monotherapy. Array BioPharma’s Filanesib (ARRY-520) has been the exception as it has demonstrated efficacy as a monotherapy for the treatment of multiple myeloma7. Filanesib is slated to be the first KSP inhibitor to be used in a Phase 3 clinical trial and will be tested as a monotherapy and in combination with standard chemotherapeutic agents8.
Some of the more recent preclinical studies using KSP inhibitors suggest that therapeutic synergy is possible when KSP inhibitors are combined with other chemotherapeutic agents9. This could potentially allow lower levels of each drug to be used, which could lead to fewer and less pronounced side effects. If these results translate into the clinical setting, we may be on the verge of seeing significant clinical benefits with KSP inhibition combo therapies.
Cytoskeleton Custom Services - Antimitotic Drug Candidates
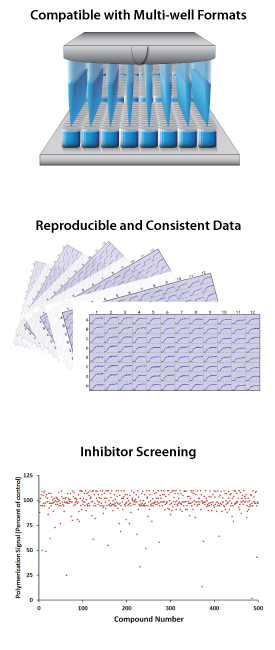
At Cytoskeleton, we have developed assays for 11 recombinant kinesin motor domains that represent 8 of the 14 recognized Kinesin subfamilies10. We offer compound screening services with individual kinesins as well as multi-motor protein panel studies. Moreover, if the kinesin assay needed for your research is unavailable, we offer custom protein expression/purification and assay development services to help move your project forward. In addition to kinesin assays, we can evaluate your compounds for their effects on microtubule polymerization. This may be a useful tool in combination with our kinesin panel screen to identify the mechanism of action for antimitotic compounds coming from phenotypic screens and/or as a useful counterscreen for kinesin inhibitor drug discovery efforts that desire to steer their SAR efforts away from compound effects on tubulin polymerization.
| Generic Name | Alternate Name | Company | Phase 1 | Phase 2 | Phase 3 | ||||
| Ispinesib | SB-715992 | Cytokinetics & GSK | 5 | 5 | |||||
| Filanesib | ARRY-520 | Array BioPharma | 3 | 4 | 1* | ||||
| Litronesib | LY2523355 | Eli Lilly & Co. | 4 | 3 | |||||
| AZD4877 | AstraZeneca | 5 | 1 | ||||||
| SB-743921 | Cytokinetics | 1 | 1 | ||||||
| MK0731 | Merck Sharp & Dohme Corp. | 1 | |||||||
| 4SC-205 | 4SC | 1 | |||||||
| ARQ621 | ARQule | 1 |
Data source = www. clinicaltrials.gov
* Planned initiation mid-20148
References
- 1. Sarli V. and Giannis A. 2008. Clin. Cancer Res. 14, 7583-7587.
- Morris P.G. and Fornier M.N. 2008. Clin. Cancer Res. 14, 7167-7172.
- Carlson K. and Ocean A.J. 2011. Clin. Breast Cancer. 11, 73-81.
- Mayer T.U., Kapoor T.M., Haggarty S.J., King R.W., Schreiber S.L., and Mitchison, T.J. 1999. Science. 286, 971-974.
- www.clinicaltrials.gov.
- Knight S.D. and Parrish C.A. 2008. Curr. Topics Med. Chem. 8, 888-904.
- Shah J.J., Zonder J., Cohen A. et al. 2011. Blood (ASH Annual Meeting Abstracts). 118, 1860.
- Owens B. 2013. Nat. Med. 19, 1550.
- Song H., Zhou S., Wang R., and Li S. 2013. Chem. Med. Chem. 8, 1736-1749.
- Lawrence C.J. et al. 2004. J. Cell Biol. 167, 19-22.
About Custom Services
Cytoskeleton, Inc. has been a reliable source of compound screening services in the areas of pre-clinical drug development programs and early compound screening in primary HTS projects, as well as secondary screening and compound target validation. We also have extensive experience in gene design and expression with an eye for producing highly purified biological active proteins. Our expertise in protein purification is the basis for the complementary skill of assay design. We have produced many functional assays for kinesins (e.g., Eg5, CenPE, MKLP2), dynein [cytoplasmic]), myosins (e.g., cardiac, smooth, skeletal, and non-muscle isoforms), small G-proteins (e.g., Rho, Arf, Ral families), tubulins (e.g., tumor, plant, and fungal origins), and actin binding proteins; many of them are multi-protein assays that might have protein complexes of 3 or more subunits, e.g., a soluble sarcomere format and the Arp2/3 complex based assay. Our experiences in antibody and ELISA technology complements the cytoskeletal and signal transduction focus. We support all of our services with a dedicated technical services department and years of laboratory experience in the fields of cell biology, cancer biology, and neuroscience.
For more details on these four main areas click on one of the following:
Compound Screening and Drug Development
ELISA and antibody development
If you have an immediate question please send an e-mail describing your requirements to tservice@cytoskeleton.com.
Have questions? Email tservice@cytoskeleton.com or call (303) 322-2254.
Exceptional and Dedicated Technical Support:
- 40+ years of combined experience
- Expertise in: Protein biochemistry, cell biology, cancer biology, and neuroscience
Product and Service Performance:
- Modules with clearly defined deliverables
- Activity tested to specification
- Shipped in desiccated chambers & guaranteed for one year
- Proven results (See citation tab above for examples)
- Satisfied clients (See examples below)
Current Clients Include:
- Merck & Co., Inc.
- Eli Lilly & Co.
- Amgen, Inc.
- Abbott Laboratories
- Pfizer, Inc.
- Astra-Zeneca plc
- GlaxoSmithKline plc
- Genentech, Inc.
- Johnson & Johnson
- Bristol-Myers Squibb