Background: Benzylguanine - SPY™ Fluorescent Substrates
Benzylguanine (BG) derivatives of Spirochrome’s SiR and SPY™ probes are now available for self-labeling SNAP-tag™* substrates.
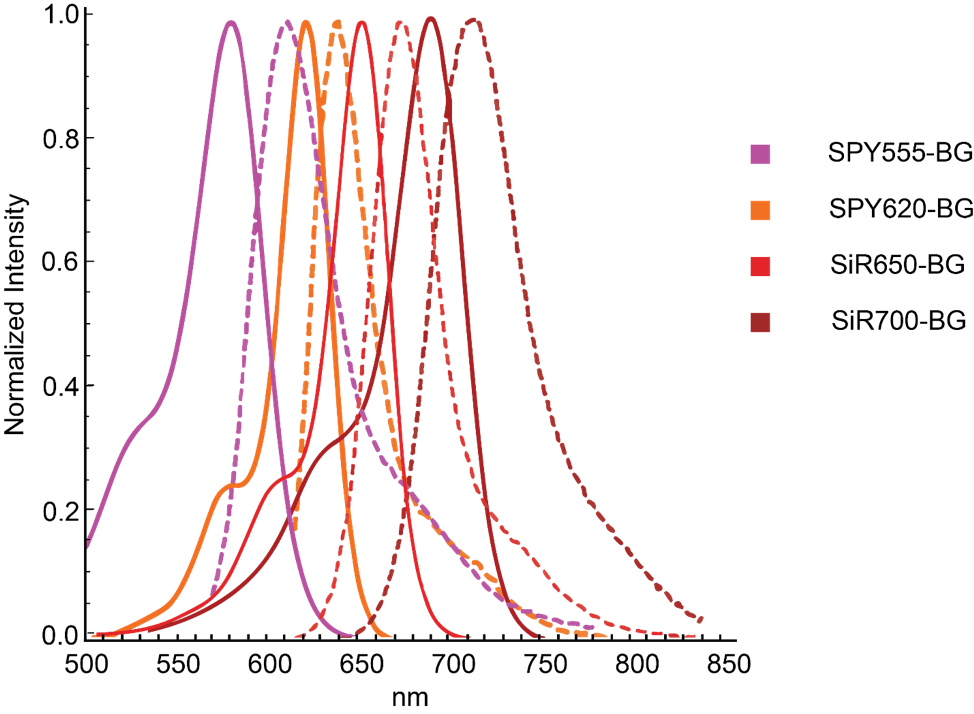
Spirochrome’s BG derivatives emit between the orange and far-red UV-ViS spectrum (Figure 1) while retaining the appealing traits of their non-BG counterparts. Spirochrome’s BG derivatives are fluorogenic, highly cell permeable, and are well suited for conventional or super resolution microscopy. One major advantage of SNAP-tag labeled proteins is the strong and stable covalent thioether bond that is not easily disrupted. These irreversible bonds create a permanent fusion between the protein of interest and the substrate even when in protein homogenates or lysates. Thus, SNAP-tagged proteins can be even loaded, detected, and quantitated with in-gel fluorescence scanning. Further, SNAP-tag fusions are versatile and can be appended to the N- or C- protein terminus avoiding disruptions in biological function.
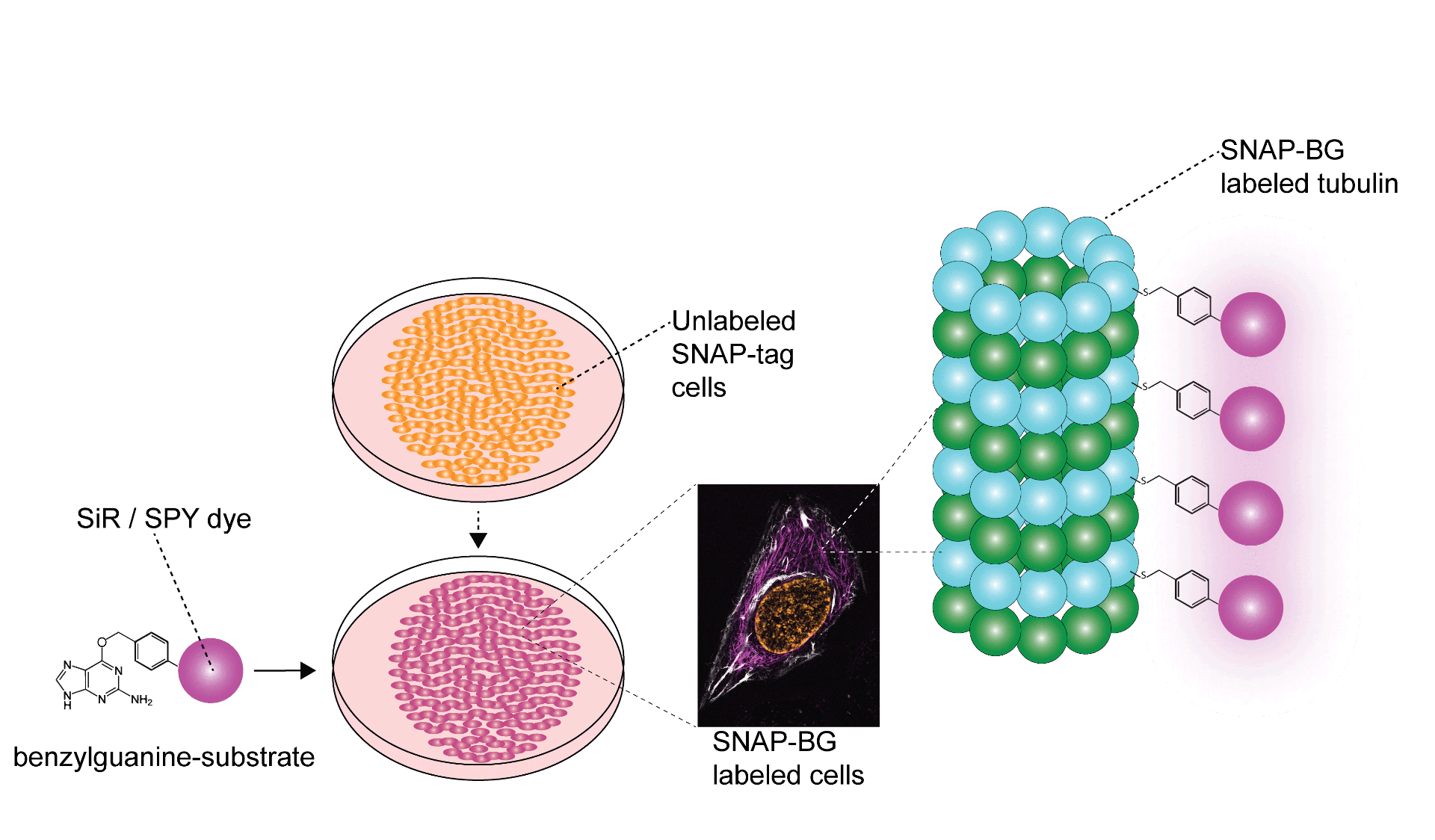
In addition to labeling the cellular surface, SNAP-tag labeling can label internal cellular structures or proteins even if located within the nucleus and ER Golgi; however, cell permeable substrates are required. Fortunately, cell permeable SPY™ and SiR probes work synergistically to label subcellular structures even internal proteins within the mitochondrial cristae1. Being compatible with STED and SIM super resolution imaging even further augments the utility of SPY™ and SiR probes, especially when analyzing spatio-temporal protein dynamics. Once cells are stably expressing fusion SNAP-tags they can be labeled by Spirochrome BG derivatives (Figure 2).
BG-Substrate Protocol
The use of BG substrates requires a suitable SNAP-tag fusion protein. Kits to produce SNAP-tag fusion proteins can be purchased from other suppliers. Once cells or tissues are stably expressing SNAP-tagged proteins, Spirochrome’s BG derivatives can be used to label expressed SNAP-tags. The general workflow and protocol are outlined below.
- Prepare DMSO stock solution.
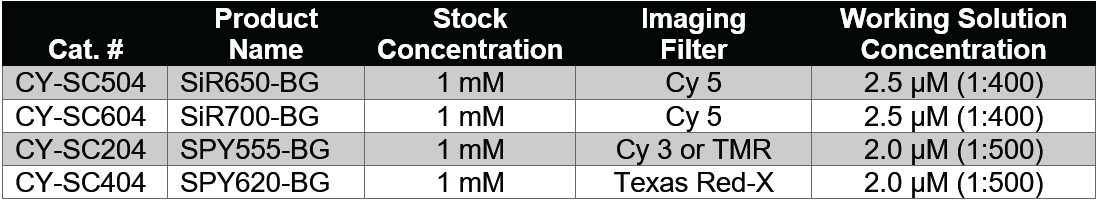
- Add 35μL of anhydrous DMSO to the BG-substrate vial to prepare a 1 mM stock solution.
- Dilute the BG-substrate DMSO stock solution according to the table below in your usual cell culture medium (e.g. DMEM + 10% fetal bovine serum) and vortex briefly.
- For best performance serum or 0.5% BSA should be present in the cell culture medium.
- The dilution is not performed in a single step, please use DMSO to prepare the intermediate dilution as using aqueous buffers to prepare the intermediate dilution will lead to the formation of BG-substrate aggregates.
- Since staining efficiency can depend on the cell line; however, we recommend using the initial concentrations in the table below that can then be optimized in further experiments until optimal staining is achieved. The usual range of BG-substrate concentration for live cell labelling is 1-10 µl.
- Use only freshly prepared staining solution, and do not use it multiple times.
- Cell preparation and staining.
- Grow cells transiently transfected or stably expressing a SNAP-tag™ fusion-protein on coverslips, glass bottom dish or glass bottom multi-well plates as usual.
- When cells have reached the desired density or expression level of the SNAP-tag™ fusion-protein, replace the culture medium by the staining solution freshly prepared under step 2 ensuring that all the cells are covered with the solution.
- Place the cells in the incubator at 37°C in a humidified atmosphere containing 5% CO2 for 30 minutes to 1h.
- After BG labeling, cells can be imaged immediately.
- Optionally, cells can be fixed by any fixation method for additional immunolabeling.
- Cell imaging.
- After labelling, the live cells can be immediately imaged without the need for washing steps. Optionally, a simple washing step consisting of replacing the labeling solution with fresh culture medium, which does not contain the BG-substrate, may improve the signal to noise ratio. If the live cells were washed before imaging, the staining duration will depend on your SNAP-tag™ fusion protein turnover rate.
Important Technical Tips
- Note that if you are transfecting cells with plasmids using Lipofectamine 3000, the lipofectamine protocol suggests 75-90% confluency at the time of transfection, followed by 2-4 days incubation prior to imaging. If using cell lines, we suggest cell confluency closer to 45-60% confluency at the point of transfection, as cells may be overgrown and begin to die prior to application of BG derivatives and imaging. This may vary according to cell line slightly.
- We recommend using newly or freshly opened and anhydrous DMSO to prepare the DMSO stock solution. In contact with air and moisture, DMSO produces decay products which can strongly reduce the shelf-life of the BG-substrate in solution, even at -20°C.
- After use, this solution should be stored at -20°C or below. Do not divide the DMSO stock solution into small aliquots. The BG-substrate is not altered by multiple freeze-thaw cycles. When stored properly, this stock solution is stable for 3 months.
*SNAP-tag™ is a registered trademark of New England Biolabs, Inc.
FAQ
1. Can BG derivatives label the organellar proteins of my model organism of interest?
Labeling organellar proteins with BG derivatives require that the protein of interest have an attached SNAP-tag for covalent attachment, and that the fluorophore of your derivative be cell permeable. Fortunately, SPY and SiR BG derivatives are highly cell permeable and have been used to label mitochondrial proteins1.
2. How do I create a fusion SNAP-tag protein?
SNAP-tag™ is a registered trademark of New England Biolabs, Inc. Requests for guidance with the creation of fusion genes and subsequent proteins should be directed to NEB.
3. How long after transfecting a cell line with protein-expressing plasmids can BG substrates be applied?
Internal testing shows that HeLa cells stably express label-ready SNAP-tags after 48 hours when using Lipofectamine 3000.