Rac proteins are small GTP-binding proteins that regulate actin-based structures such as lamellipodia, membrane ruffles, and dorsal ruffles, enabling dynamic remodeling of the cytoskeleton. They are essential for processes including cell migration, adhesion, and growth factor–mediated signaling.
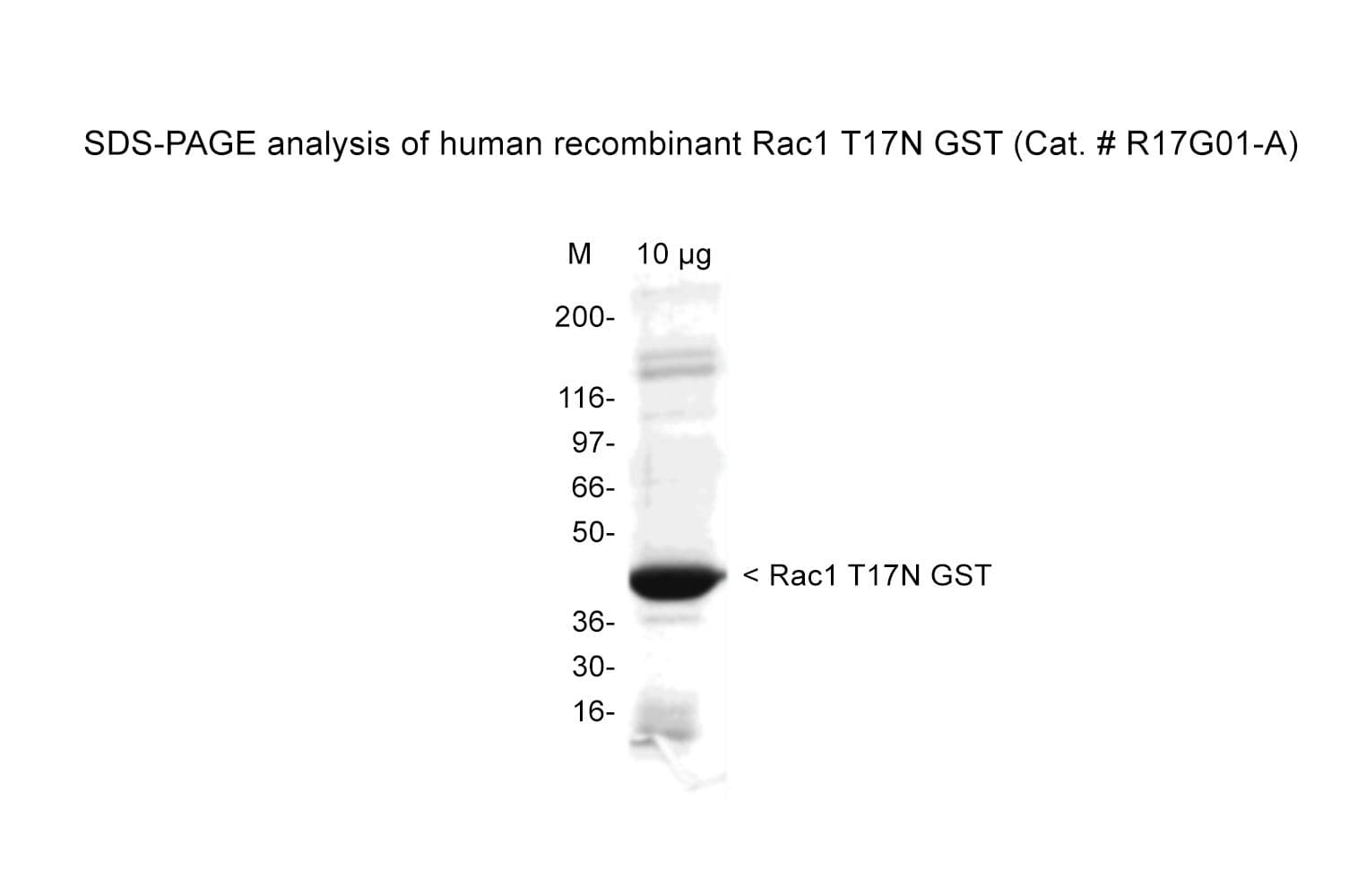
The dominant negative form of human Rac1 protein is produced in a bacterial expression system. This protein has a threonine to asparagine substitution at amino acid 17. It exists in a stabilized nucleotide-free or GDP-bound conformation and has a high affinity for GEFs but cannot be activated by them.
Protein purity is assessed by scanning densitometry of Coomassie Blue-stained protein on a 4-20% polyacrylamide gel. Purity was determined to be ≥80% pure.
The biological activity of R17G01 is determined by testing its ability to inhibit Vav2 CS-GE06 catalyzed nucleotide exchange on wild-type Rac1. Quality control standards require that, in the standard assay, nucleotide exchange on wild type Rac1 is inhibited 30-50% by equimolar amounts of R17G01.
Cat. #R17G01-A

© 2026 Cytoskeleton, Inc All Rights Reserved.