Cdc42 is a small GTP-binding protein in the Rho family that regulates cell polarity, cytoskeletal organization, and signal transduction. It is best known for controlling the formation of filopodia—thin, actin-rich projections that help cells sense their environment and guide directional movement.
The dominant negative form of the human Cdc42 protein is produced in a bacterial expression system. This protein has a threonine to asparagine substitution at amino acid 17. It exists in a stabilized nucleotide-free or GDP-bound conformation and has a high affinity for GEFs but cannot be activated by them.
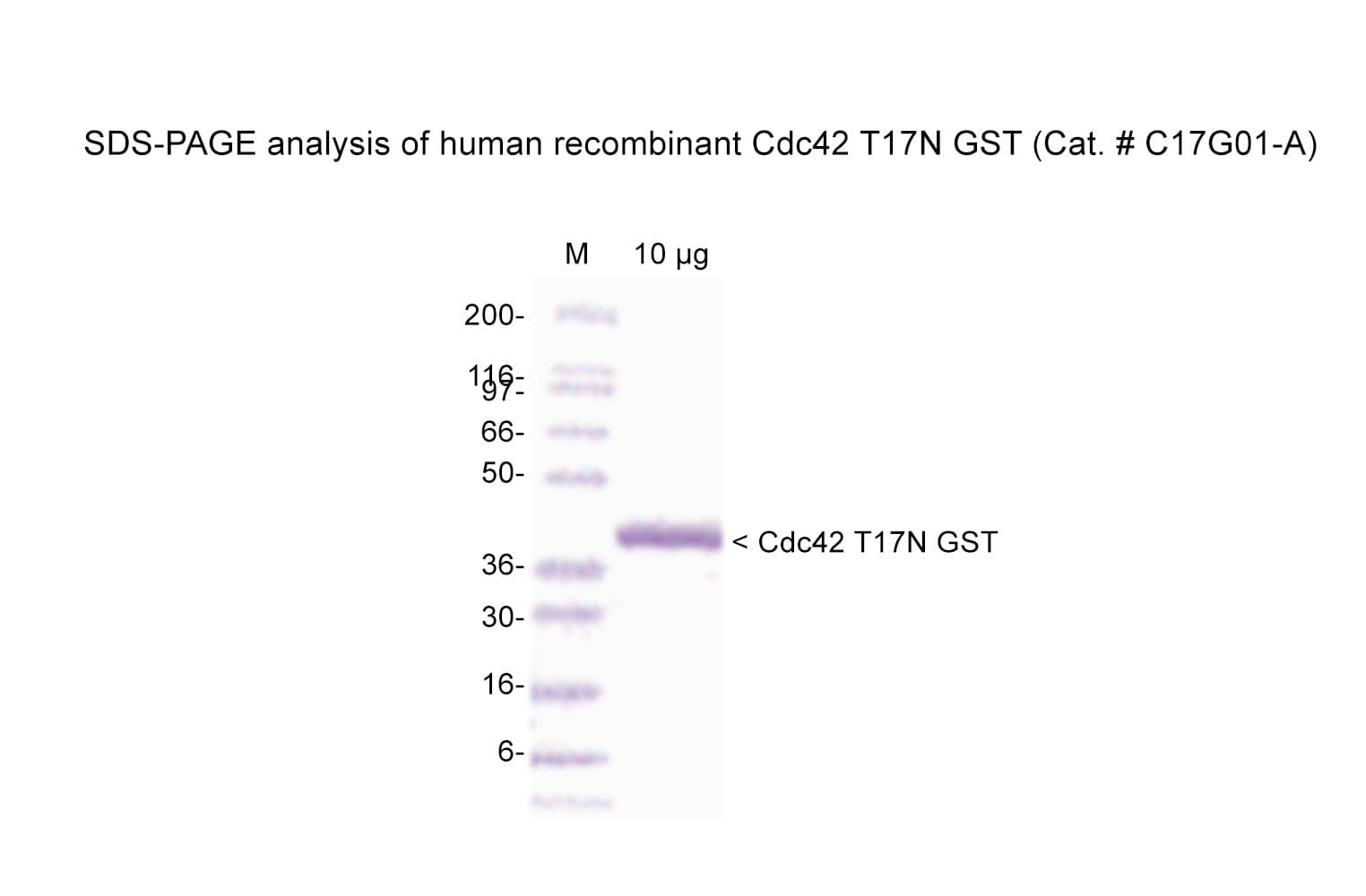
Protein purity is assessed by scanning densitometry of Coomassie Blue-stained protein on a 4-20% polyacrylamide gel. Purity was determined to be ≥90% pure.
The biological activity of C17G01 is determined by testing its ability to inhibit hDbs GE01 catalysed nucleotide exchange on wild-type Cdc42. Quality control standards require that, in the standard assay, nucleotide exchange on wild-type Cdc42 is inhibited 30-50% by equimolar amounts of C17G01.
Cat. #C17G01-A