CT03, part of the G-Switch™ line, is a recombinant form of C3 transferase from Clostridium botulinum. C3 transferase is an ADP-ribosyl transferase that selectively ribosylates RhoA, -B, and -C proteins in the effector-binding domain on asparagine 41, rendering them inactive. Produced in a bacterial expression system, CT03 is a 24 kDa recombinant protein with an N-terminal six-histidine tag. Supplied as a lyophilized powder.
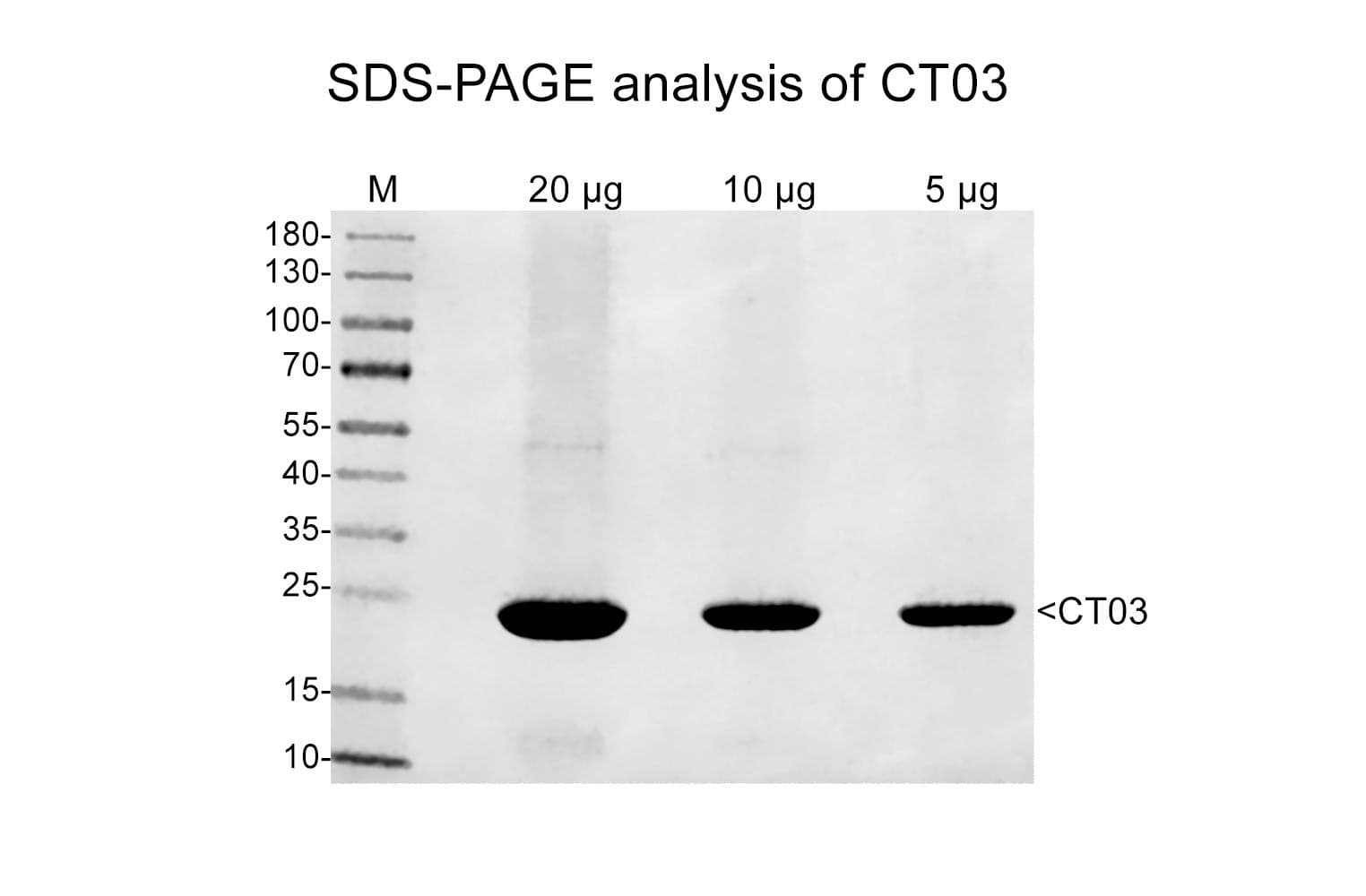
Protein purity is assessed by scanning densitometry of Coomassie Blue stained protein on a 4-20% polyacrylamide gel. Purity was determined to be >80%.
The biological activity of CT03 is demonstrated by its ability to ADP ribosylate native Rho in human platelet extracts. A standard biological assay for monitoring the ADP ribosylation of Rho consists of an in vivo ribosylation reaction followed by non-denaturing gel electrophoresis and Western blot analysis. Stringent quality control ensures that > 80% of native Rho protein is ADP ribosylated by the recombinant C3 transferase.
Cat. #CT03