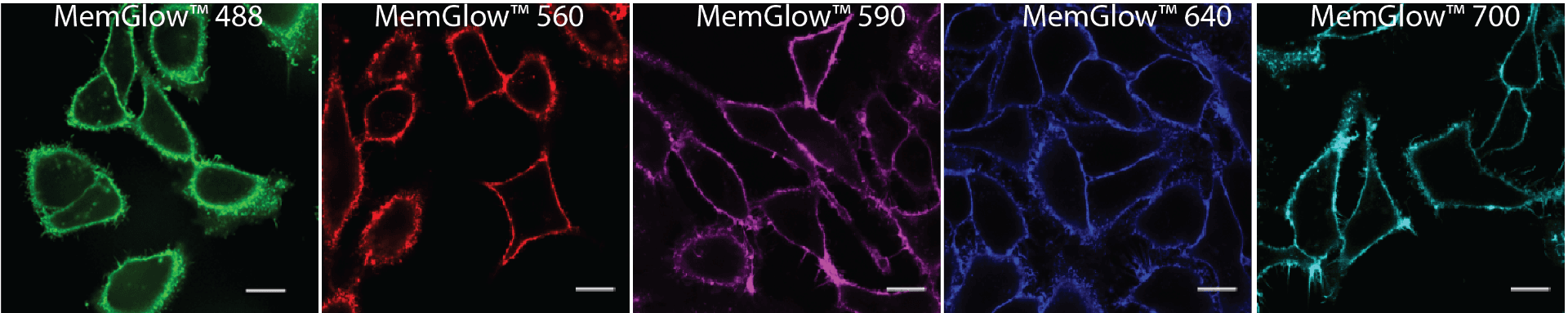
Plasma membrane staining techniques using fluorescent probes
The eukaryotic plasma membrane (PM) is a lipid bilayer organized into a continuous barrier that separates the cellular milieu from the extracellular space. The physical barrier provided by the plasma membrane also functions as biological scaffolding for proteins that mediate signal transduction or elicit cellular responses, such as Ras1, to extracellular events occurring at the cell’s surface. In addition to these functions, the PM also acts as an intercellular hub via release and reception of encapsulated biomolecules known as extracellular vesicles (EVs)2.
MemGlow™
The MemGlow™ family of probes (Figure 1) offer a new solution for staining phospholipid membranes and consists of 5 fluorogenic cyanine-based plasma membrane probes with a wide spectral range that includes near-infrared (Abs.max 499-689 nm, Em.max 506-713)3.
The MemGlow™ probes possess many of the desirable characteristics of their original carbocyanine or BODIPY parent dyes, while exhibiting greater efficiency than competing labeling products in some applications. The advantages conferred to MemGlow™ 560 and 640 probes are owed to their chemical structures, based on a symmetrical cyanine structure, which were further enhanced by the chemical attachment of two zwitterionic anchors. Similarly, MemGlow™ 488 is an amphiphilically tuned BODIPY probe enhanced via the addition of zwitterionic anchors to improve membrane specificity beyond the ability of the parent BODIPY dye. Zwitterionic anchors promote the dissociation and intercalation of probe aggregates into plasma membranes while simultaneously enhancing their retention (Figure 2).
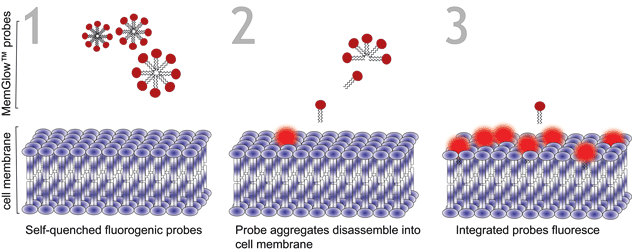
MemGlow™ application is simple. When MemGlow™ probes are introduced into aqueous media, the amphiphilic probes form self-quenching aggregates until contact with a plasma membrane elicits their dissociation and dispersal into the lipid bilayer. Once integrated, the fluorogenic probes are ready for bioimaging. From MemGlow™ 488 to MemGlow™ 700, MemGlow™ probes produce intense fluorescence many times greater than background and exhibit signal-to-background ratios (S/B) between ~20 and 3.4, respectively3,4. MemGlow™ probes are a technological breakthrough and superior to many contemporary stains such as: 1) PKH dyes which require a convoluted protocol and alter normal biology , 2) long-chain carbocyanine dyes which possess variable non-uniform dye-dependent characteristics and are incompatible with Triton X-100 permeabilization, and 3) WGA Alexa-conjugated solutions which require uniformity of cell surface glycan-expression in order to produce consistent results. MemGlow™ labeling is extremely efficient and even nanomolar concentrations enable visualization of intercellular filopodia and nanotubes (Figure 3A and 3B).
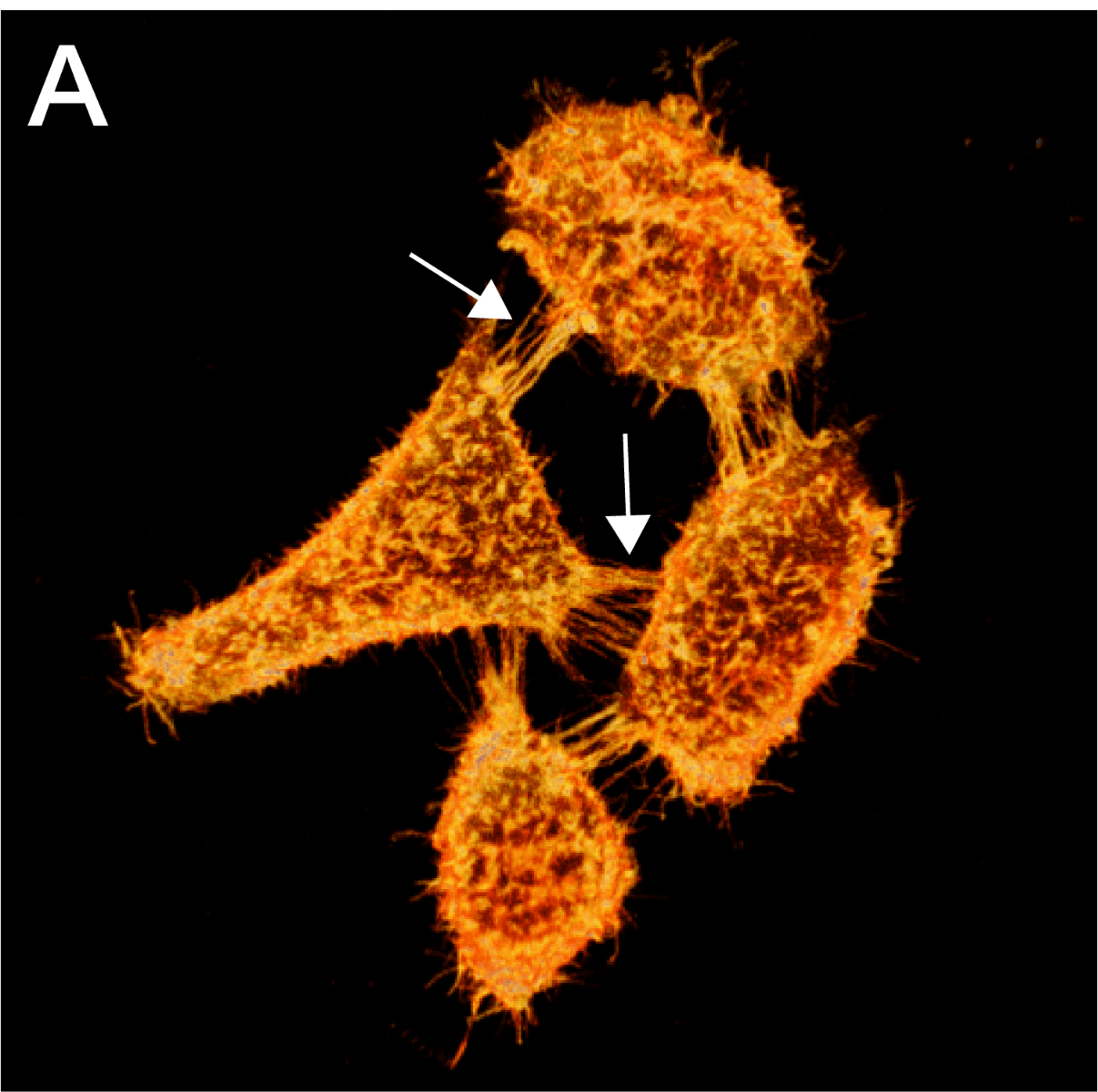
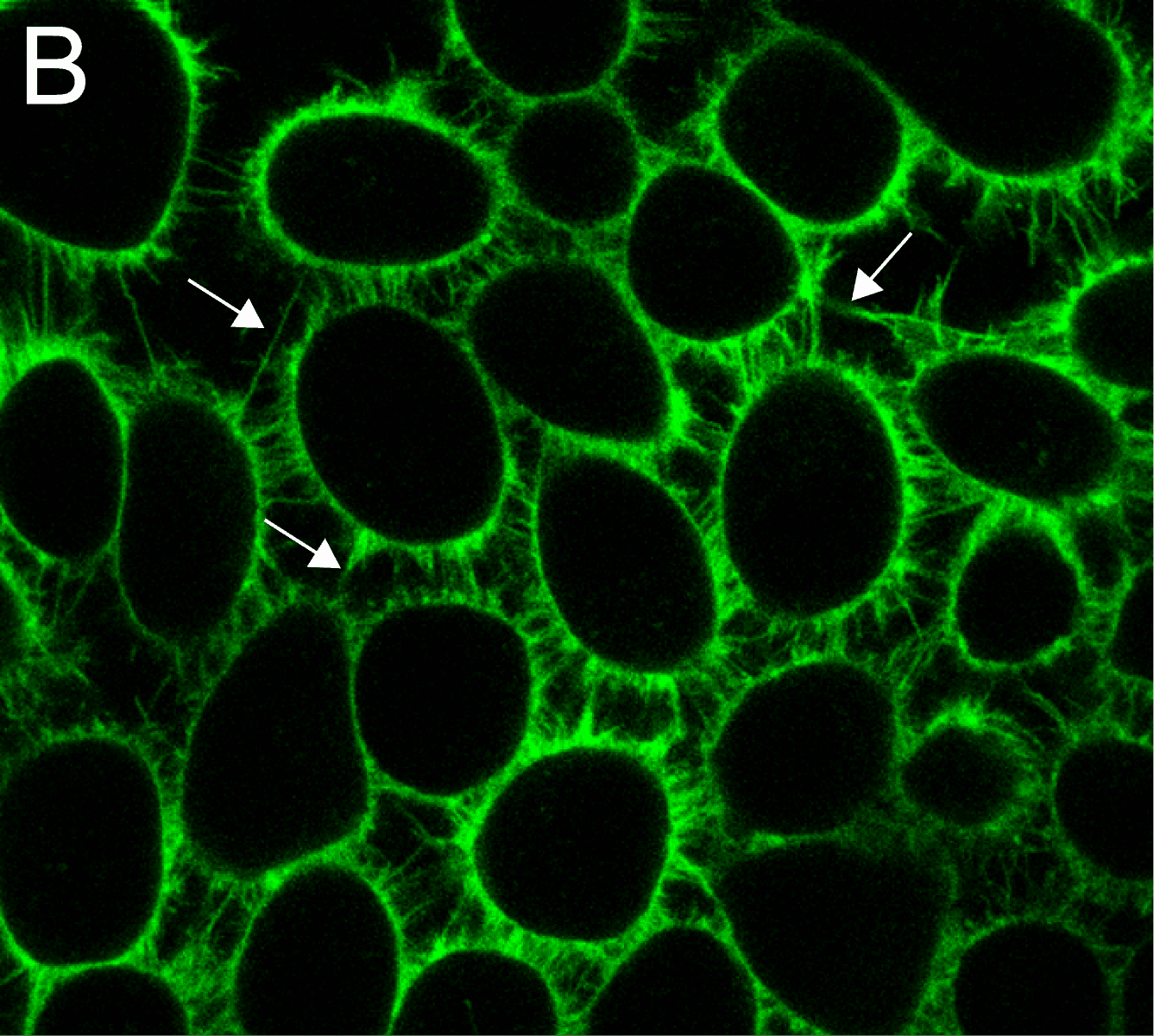
What makes MemGlow™ the leader in membrane labeling?
1) MemGlow™ probes are fluorogenic.
When MemGlow™ probes are introduced into aqueous media the amphipathic probes form self-quenching aggregates until contact with a plasma membrane elicits their dissociation and dispersal into the lipid bilayer. Once integrated, the fluorogenic probes are ready for bioimaging. This feature of MemGlow™ makes the application more straightforward than dyes like Sigma Aldrich’s PKH product line which requires special conditions to remove excess dye prior to imaging.
2) The application of MemGlow™ probes to label the cellular PM of cells is not dependent upon homogenous expression of surface carbohydrates.
Labeling of live HeLa and KB cell PMs with 20 nM MemGlow™ was 3-fold more homogenous and efficient than Thermo Fisher Scientifics’s WGA-Alexa Fluor 488 (1 mg/ml); possible due to cell membrane overlap obscuring PM carbohydrates required for WGA-binding³. MemGlow is the better choice when working with confluent cells.
3) MemGlow™ probes are compatible with co-labeling requiring permeabilization.
MemGlow™ probes have been successfully used in co-labeling experiments requiring Triton-X up to 0.1%³.
4) MemGlow™ probes are highly specific for plasma membranes when applied to fixed cells.
When applied to 4% PFA-fixed HeLa and KB cells MemGlow™ was more specific for the plasma membrane compared to Thermo Fisher Scientifics’s WGA-Alexa Fluor 488 which also stained the perinuclear envelope and additional organelles³.
5) MemGlow™ probes effectively label the dendritic spines of live neurons without the use of transgenic cell lines, transgenic animals, or transfection steps.
MemGlow™ preferentially labeled the plasma membrane of live neurons, relative to glial cells, without the deleterious effects associated with transfection-based methods³.
6) MemGlow™ probes are highly specific for the plasma membrane and are not rapidly endocytosed.
MemGlow™ 560 and 640 probes provide superior and more consistent labeling of live HeLa and KB cell PMs with little internalization even after 90 minutes³.
7) The MemGlow™ product line includes far-red probes.
Far-red dyes possess a longer absorption and excitation wavelengths above 650 nm. This wavelength spectra of bio-imaging dyes produces ideal staining characteristics such as low background, the ability to detect emission in deeper tissue, high-resistance to photobleaching, and can be excited without co-excitation and visualization of green auto fluorescing biomolecules5,6. While intracellular relocation of membrane probes in live cells is inevitable, due to normal plasma membrane turnover, the far-red versions of the MemGlow™ probes are structurally distinct from the standard probes, and exhibit greater lipophilicity conferring resistance to endocytosis³.
MemGlow™ 590 and 700 are red-shifted probes that are structurally distinct from their standard MemGlow™ counterparts and resist endocytosis when labeling live HeLa and KB cell PMs and show little probe internalization even after 90 minutes³.
8) MemGlow™ probes are highly efficient at low concentrations enabling long-term visualization studies and provide superior cellular PM labeling compared to contemporary dyes that require 250-fold greater concentrations.
Fixed primary hippocampal neurons from mice were labeled by 5 µg/ml WGA-647, 5 µM DiD, mCling-647, and 200 nM MemGlow™ 590³. WGA-Alexa Fluor 647 staining was mostly homogenous for the plasma membrane; however, there were distinct unlabeled sections along the cell periphery. DiD performed better than WGA-Alexa Fluor 647, however; distinct cell-to-cell variation of PM labeling was observed. mCling labeling of the PM was inconsistent, quickly internalized into intracellular vesicles, and lethal at concentrations above 2 µM. To contrast, MemGlow™ 590 effectively and efficiently labeled the PM of the neuronal body and neuronal protrusions at 200 nM concentrations³.
9) MemGlow™ 590 is capable of blinking and thus is compatible with Stochastic Optical Reconstruction Microscopy (STORM).
HeLa cells were fixed in 4% PFA prior to labeling with 20 nM of MemGlow™ 590 and high-resolution images were reconstructed from 6000-10000 frames³.
10) MemGlow™ 560, 590, 640, and 700 probes are compatible two-photon excitation (TPE) microscopy.
MemGlow™ 560, 590, 640, and 700 are able to achieve up to a 1200 Goeppert-Mayer cross section in DMSO, and successfully produce high-signal and low-noise images of live KB cell plasma membranes without washing³.
Citations
- Zhou, Y., Prakash, P., Gorfe, A. A. & Hancock, J. F. Ras and the Plasma Membrane: A Complicated Relationship. Cold Spring Harb. Perspect. Med. 8, (2018).
- Van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology vol. 19 213–228 (2018).
- Collot, M. et al. MemBright: A Family of Fluorescent Membrane Probes for Advanced Cellular Imaging and Neuroscience. Cell Chem. Biol. 26, 600-614.e7 (2019).
- Collot, M., Boutant, E., Lehmann, M. & Klymchenko, A. S. BODIPY with Tuned Amphiphilicity as a Fluorogenic Plasma Membrane Probe. Bioconjug. Chem. 30, 192–199 (2019).
- Ni, Y. & Wu, J. Far-red and near infrared BODIPY dyes: synthesis and applications for fluorescent pH probes and bio-imaging. Org. Biomol. Chem. 12, 3774 (2014).
- Yuan, L., Lin, W., Zheng, K., He, L. & Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chemical Society Reviews vol. 42 622–661 (2013).
For more information on Cytoskeleton's selection of probes click below
MemGlow™ 488: Fluorogenic Membrane Probe (A Member Of The MEMBRIGHT™ Family) (Cat. # MG01)
MemGlow™ 560: Fluorogenic Membrane Probe (A Member Of The MEMBRIGHT™ Family) (Cat. # MG02)
MemGlow™ 590: Fluorogenic Membrane Probe (A Member Of The MEMBRIGHT™ Family) (Cat. # MG03)
MemGlow™ 640: Fluorogenic Membrane Probe (A Member Of The MEMBRIGHT™ Family) (Cat. # MG04)
MemGlow™ 700: Fluorogenic Membrane Probe (A Member Of The MEMBRIGHT™ Family) (Cat. # MG05)