+3
Tubulin protein T240 is labeled on random surface lysines using HiLyte™647 activated ester;
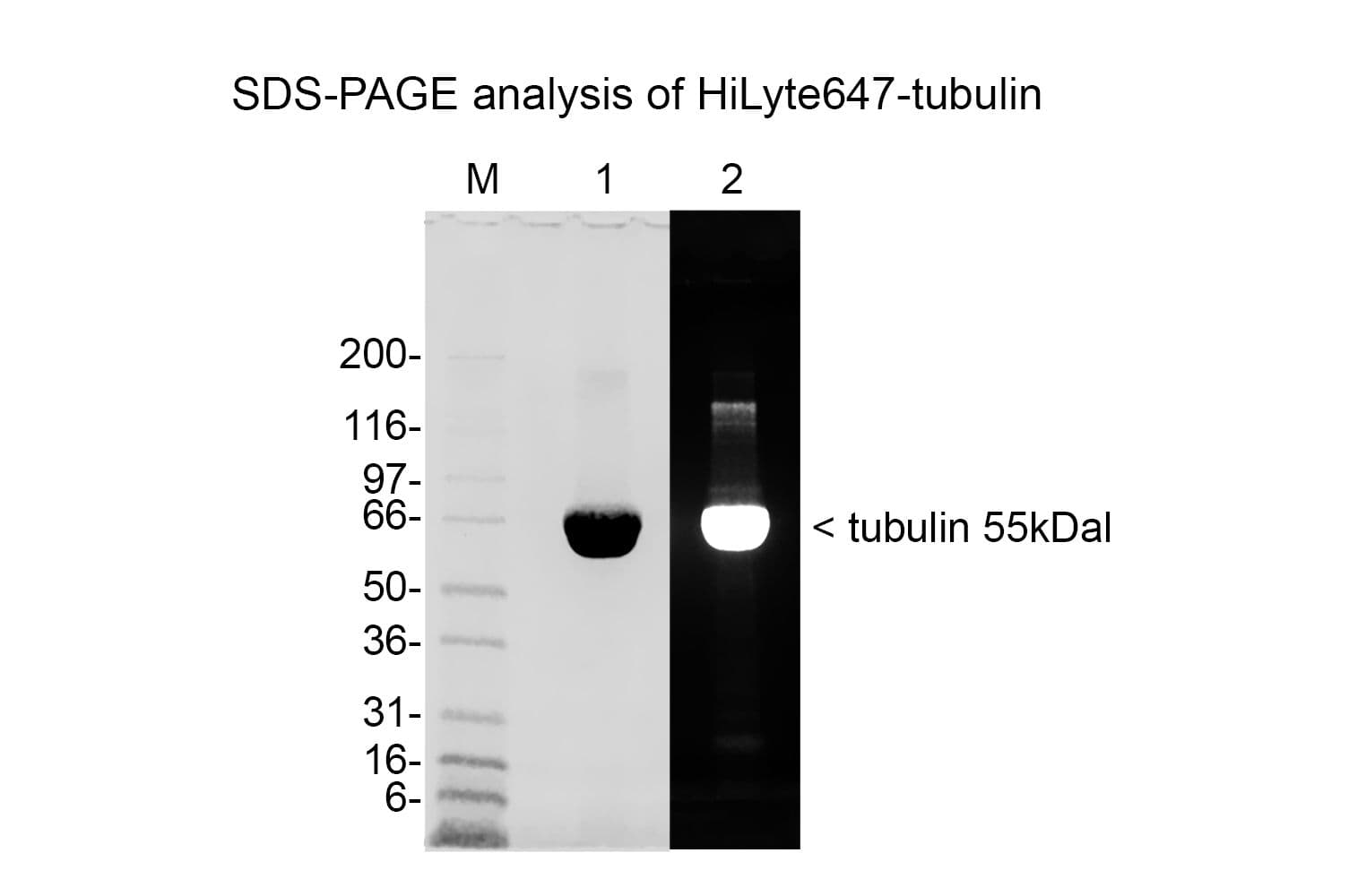
Protein purity is assessed by scanning densitometry of Coomassie Blue stained protein on a12% polyacrylamide gel. Purity is determined to be >99% pure.
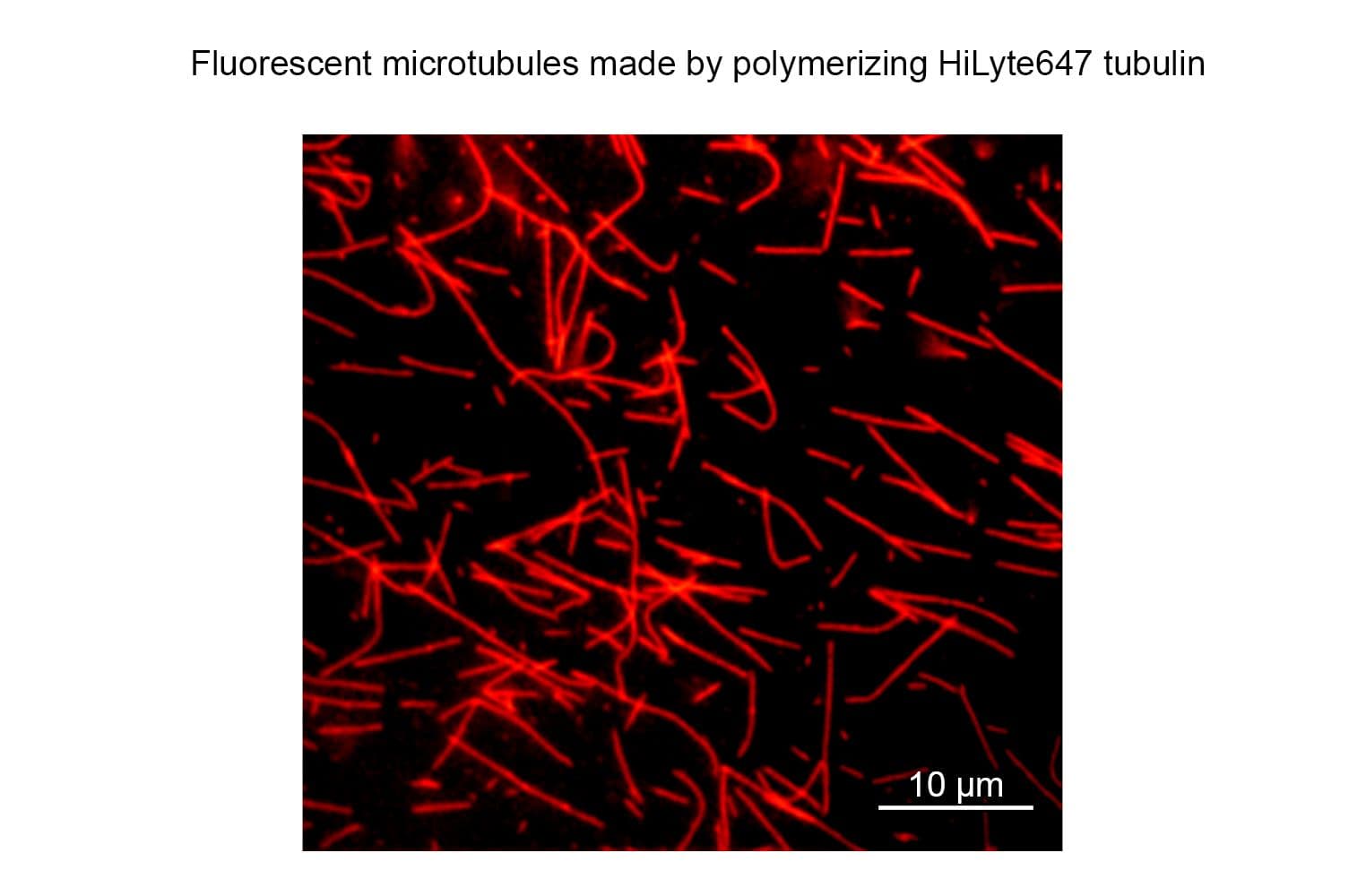
TL670M tubulin retains high biological activity, with >80% polymerizing into microtubules in vitro, as confirmed by a spin-down assay. This performance is comparable to that of unmodified tubulin.
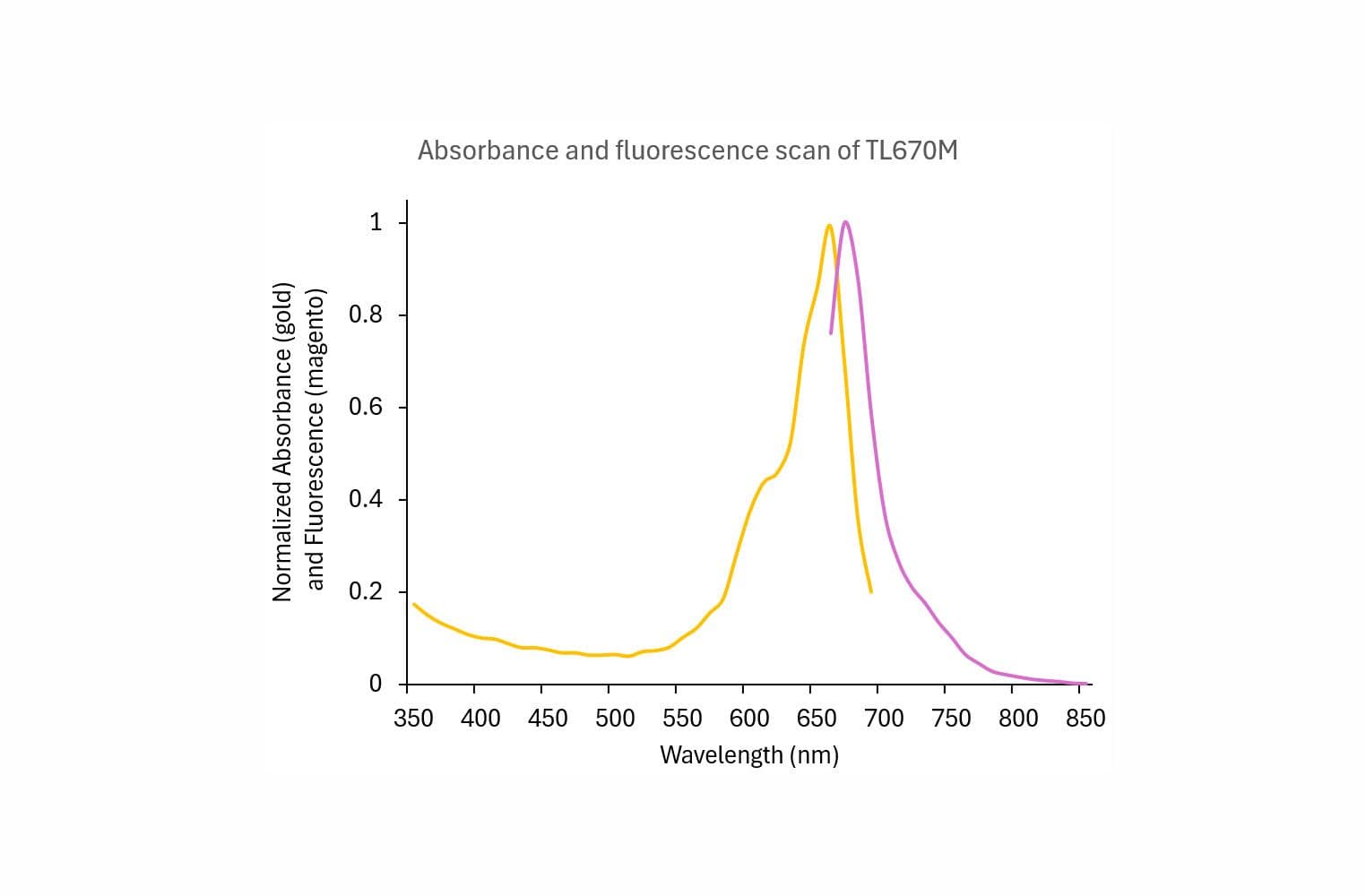
Cat. #TL670M