Tubulin protein (fluorescent HiLyte 647): porcine brain
HiLyte Fluor™ 647labeled microtubules formed from HiLyte Fluor™ 647 labeled tubulin.
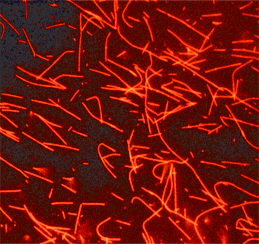
Product Uses Include
- Laser based applications
- Monitoring microtubule dynamcs in living cells
- Speckle microscopy
- Formation of fluorescent microtubules
- Microscopy studies of MAP and microtubule associated motor activities
- Nanotechnology
Material
Porcine brain tubulin (>99% pure, see Cat. # T240) has been modified to contain covalently linked HiLyte Fluor™ 647 (HiLyte Fluor is a trademark of Anaspec Inc, CA) at random surface lysines. An activated ester of HiLyte Fluor™ 647 was used to label the protein. Labeling stoichiometry was determined by spectroscopic measurement of protein and dye concentrations (dye extinction coefficient when protein bound is 250,000M-1cm-1). Final labeling stoichiometry is 0.2 to 0.7 dyes per tubulin heterodimer. HiLyte Fluor™ 647 labeled tubulin can be detected using a filter set of 600-630 nm excitation and 660-680 emission. HiLyte Fluor™ 647 tubulin is in a versatile, stable and easily shipped format. It is ready for micro-injection or in vitro polymerization. Cytoskeleton, Inc. also offers AMCA (Cat. # TL440M), HiLyte Fluor™ 488 (Cat. # TL488M), rhodamine (Cat. # TL590M), X-rhodamine (Cat. # TL620M) labeled tubulins.
Purity
The protein purity of the tubulin used for labeling is determined by scanning densitometry of Coomassie Blue stained protein on a 4-20% polyacrylamide gel. The protein used for TL670M is >99% pure tubulin (Figure 1 A). Labeled protein is run on an SDS gel and photographed under UV light. Any unincorporated HiLyte Fluor™ 670 dye would be visible in the dye front. No fluorescence is detected in the dye front, indicating that no free dye is present in the final product (Figure 1 B).
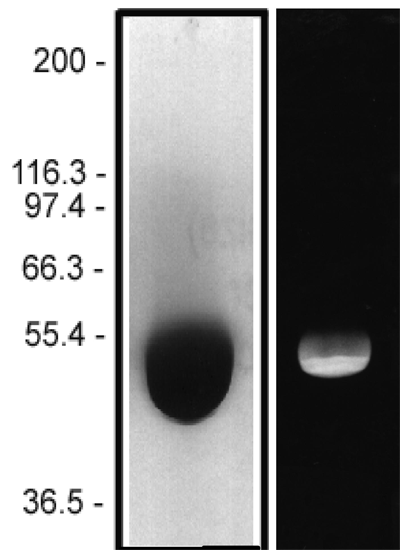
Figure 1: HiLyte Fluor™ 647 tubulin protein purity determination. A 50 µg sample of unlabeled tubulin protein was separated by electrophoresis in a 4-20% SDS-PAGE system and stained with Coomassie Blue (A). Protein quantitation was performed using the Precision Red Protein Assay Reagent (Cat. # ADV02). 20 µg of the same protein sample was run in a 4-20% SDS-PAGE system and photographed directly under 525-625nm illumination (B).
Biological Activity
The biological activity of HiLyte Fluor™ 647 tubulin is assessed by a tubulin polymerization assay. To pass quality control, a 5 mg/ml solution of HiLyte Fluor™ 647 labeled tubulin in G-PEM plus 5% glycerol must polymerize to >85%. This is comparable to unlabeled tubulin under identical conditions.
For product Datasheets and MSDSs please click on the PDF links below. For additional information, click on the FAQs tab above or contact our Technical Support department at tservice@cytoskeleton.com
Question 1: Can HiLyte Fluor™ 647-labeled tubulin (Cat. # TL670M) be used to monitor tubulin dynamics in living cells?
Answer 1: Yes, all of Cytoskeleton’s fluorescently-labeled tubulins, including TRITC rhodamine-tubulin can be micro-injected into cells to study tubulin localization and dynamics in living cells. Please see the brief protocol on the product datasheet (Cat. # TL670M and these papers for guidance on micro-injecting cells with fluorescently-labeled proteins (Smilenov et al., 1999. Focal adhesion motility revealed in stationary fibroblasts. Science. 286, 1172-1174; Lopez-Lluch et al., 2001. Protein kinase C-d C2-like domain is a binding site for actin and enables actin redistribution in neutrophils. Biochem. J. 357, 39-47; Lim and Danuser, 2009. Live cell imaging of F-actin dynamics via fluorescent speckle microscopy (FSM). J. Vis. Exp. 30, e1325, DOI: 10.3791/1325;
Question 2: What is the best way to store HiLyte Fluor™ 647-labeled tubulin to maintain high activity?
Answer 2: The recommended storage condition for the lyophilized tubulin product is 4°C in the dark with desiccant to maintain humidity at <10%. Under these conditions the protein is stable for 6 months. Lyophilized protein can also be stored desiccated at -70°C where it will be stable for 6 months. However, at -70°C the rubber seal in the lid of the tube could crack and allow in moisture. Therefore we recommend storing at 4°C. If stored at -70°C, it is imperative to include desiccant with the lyophilized protein if this storage condition is utilized. After reconstituting the protein as directed, the concentrated protein in G-PEM buffer should be aliquoted, snap frozen in liquid nitrogen and stored at -70°C (stable for 6 months). NOTE: It is very important to snap freeze the tubulin in liquid nitrogen as other methods of freezing will result in significantly reduced activity. Defrost rapidly by placing in a room temperature water bath for 1 min. Avoid repeated freeze/thaw cycles.
If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com


