+3
Kit contents (sufficient for 96 assays)
Equipment & materials required
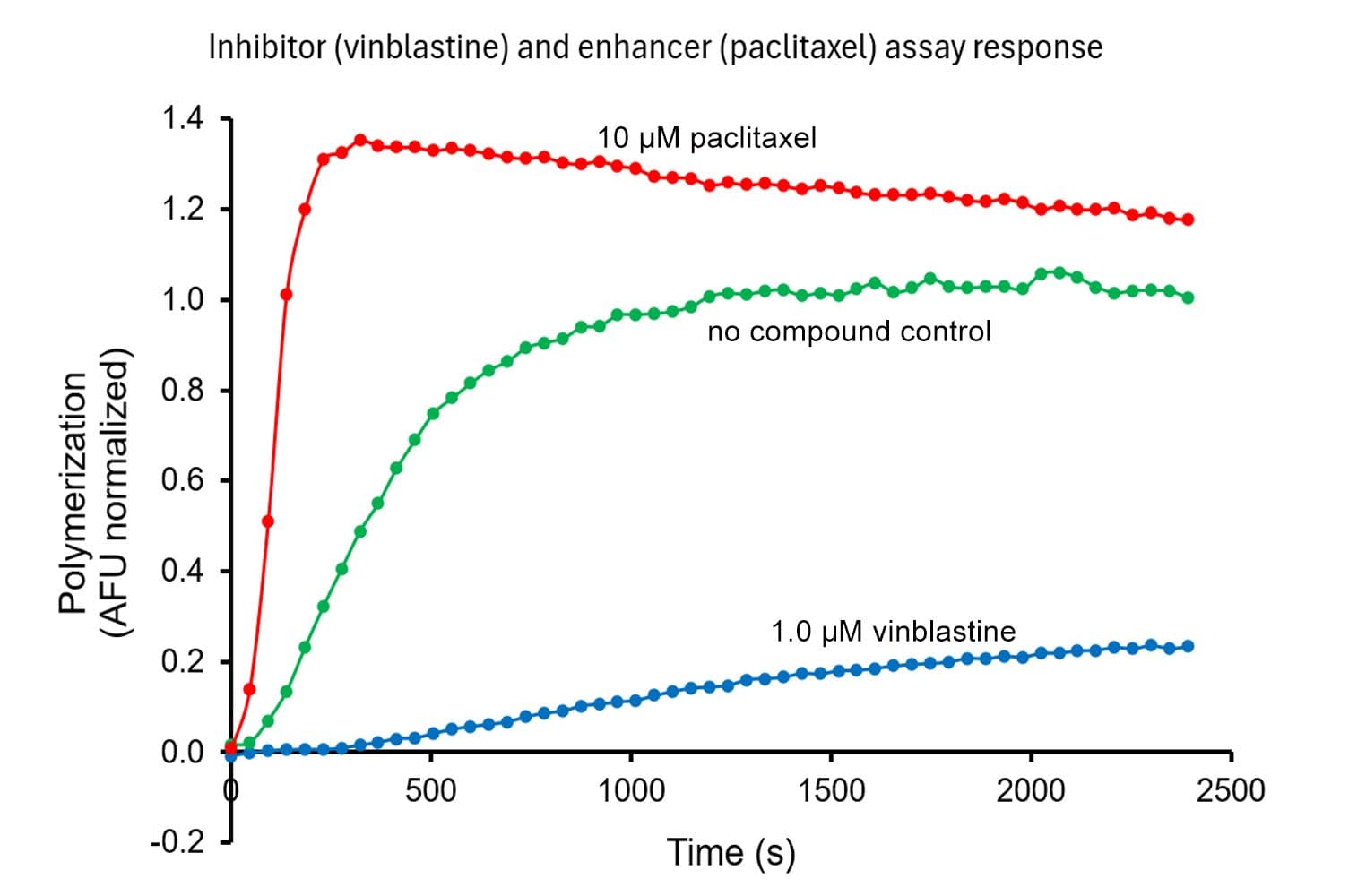
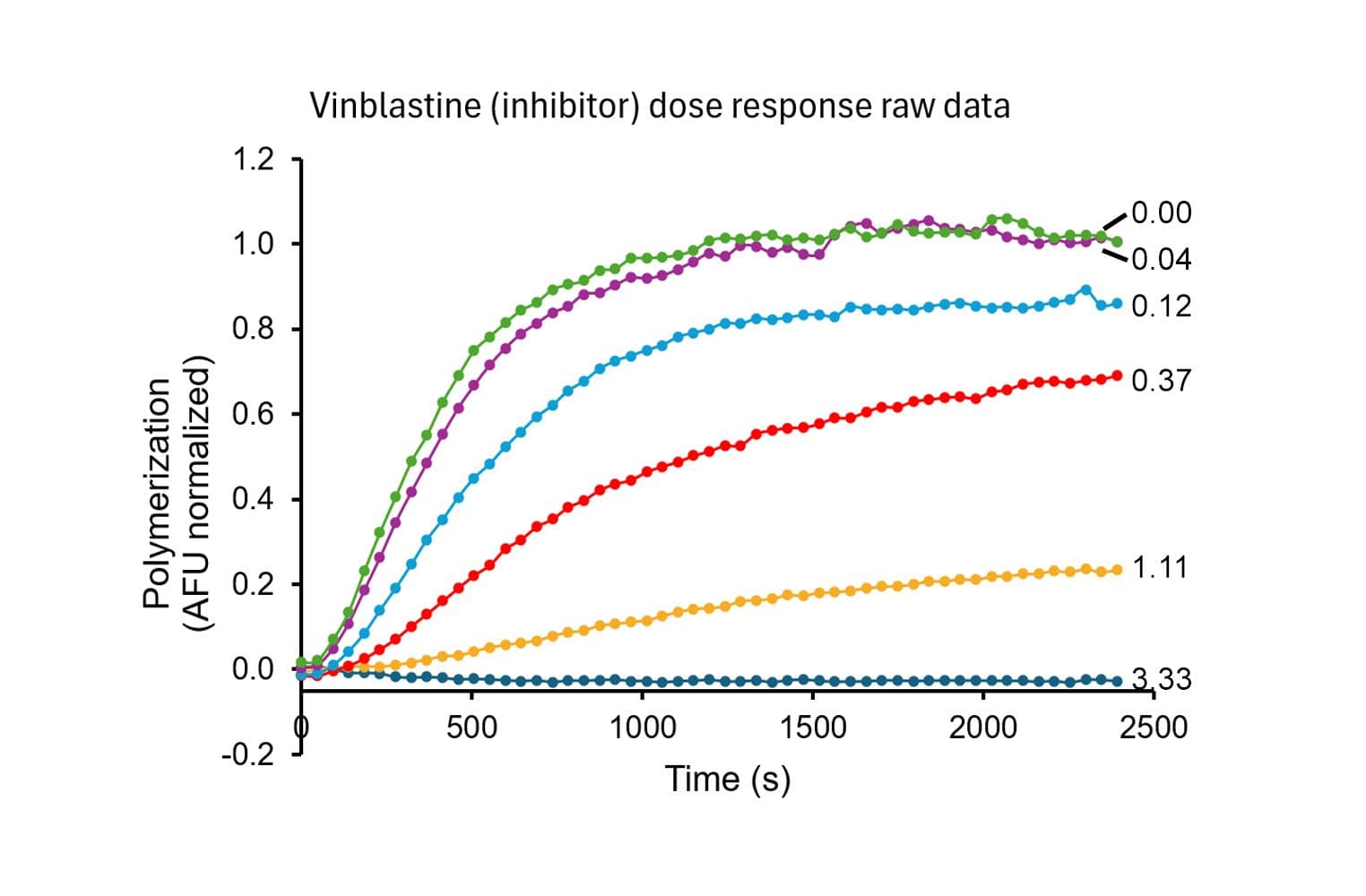
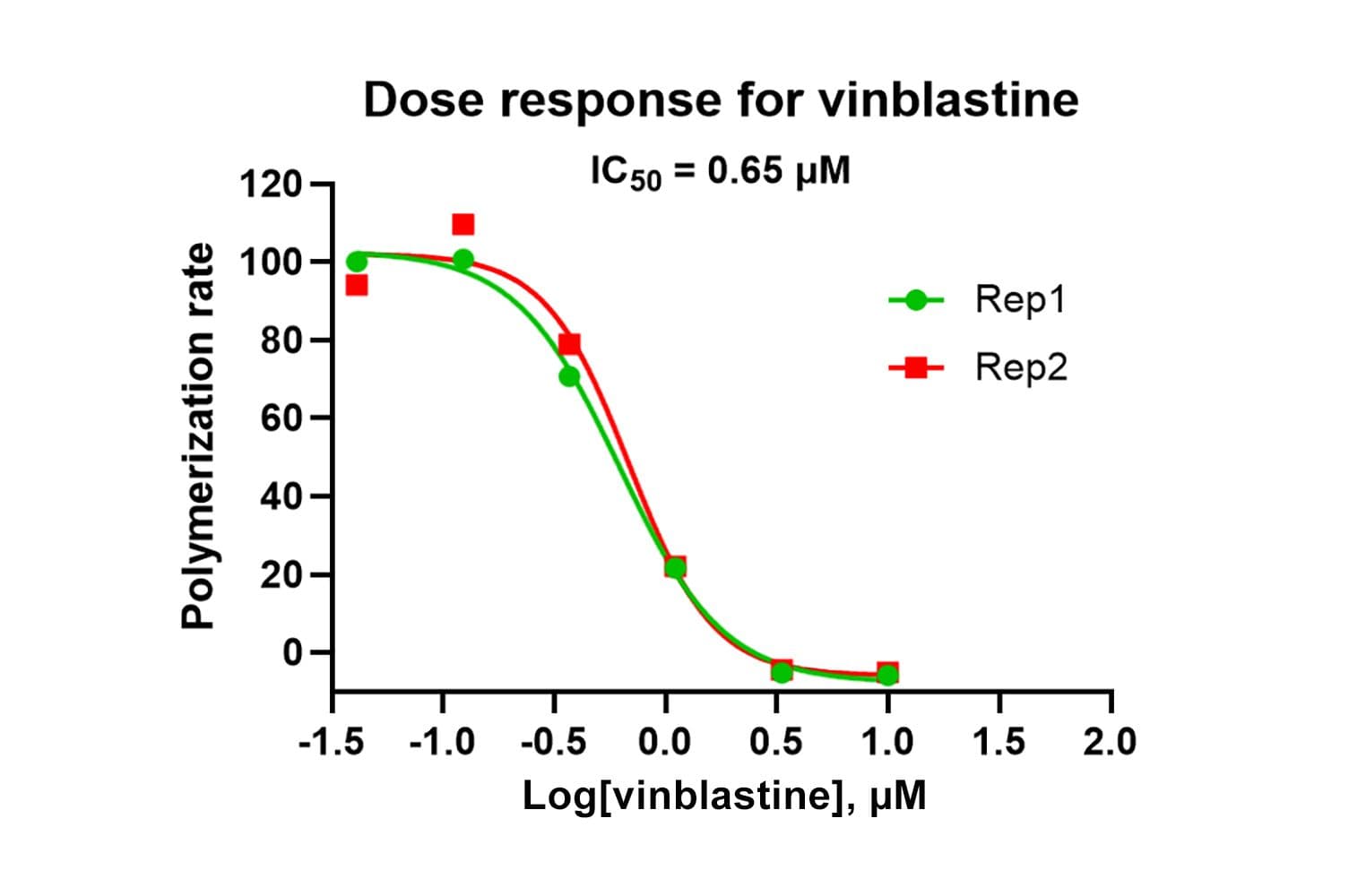
This assay measures tubulin polymerization dynamics using a fluorescent reporter that incorporates into microtubules as they form. It is designed to monitor the real-time kinetics of microtubule assembly and disassembly, which is critical for understanding cellular functions and screening pharmacological agents.
Key features
Cat. #BK011P