Myosin-driven actin-microtubule networks exhibit self-organized contractile dynamics
- By Cytoskeleton Inc. - Tubulin News
- Jun 22, 2021

Myosin-driven actin-microtubule networks exhibit self-organized contractile dynamics
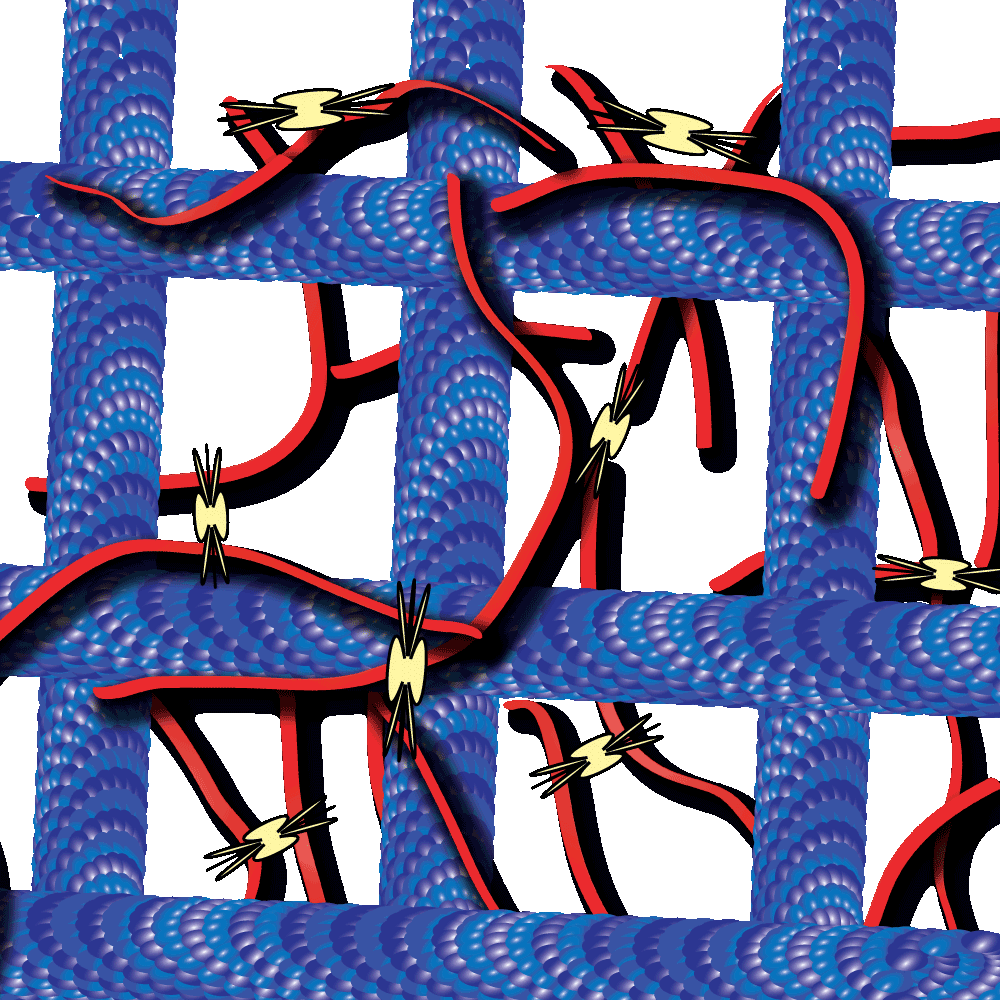
Cytoskeleton proteins, actin and tubulin, are two of the most well-characterized proteins and the networks they form have been actively studied over the past decade. Interestingly, these actin and tubulin networks interact extensively in cells, yet our understanding of how these networks affect the other’s dynamics is just beginning to come to light. Work by Dr. Ross and Dr. Robertson-Anderson has led the way towards unmasking this mystery. In their recent study these groups sought to determine how the interactions between actin and microtubule networks impact actomyosin activity. They utilized cutting-edge technologies like particle image velocimetry and dynamic differential microscopy to measure the network dynamics of actin and microtubules that are co-entangled in the presence of myosin II. Interestingly they observed that microtubules facilitated organized contraction of actomyosin networks that were otherwise disjointed and displayed disordered dynamics. Importantly, they also observed that these co-entangled networks can undergo ballistic contraction with indistinguishable characteristics. Furthermore, they observed several other unique properties of these co-entangled actin-microtubule networks which collectively suggest that the interaction between these networks may be important in tuning actomyosin activity in cells. Cytoskeleton’s purified actin (Cat. # AKL99), tubulin (Cat. # T240), and myosin (Cat. # MY02) proteins were useful tools that aided in this study to better understand how these cytoskeletal networks co-regulate their dynamic properties.
Link to Citation:
Related Products / Products Used:
Myosin II protein: rabbit skeletal muscle (Cat. # MY02)
Actin protein (>99% pure): rabbit skeletal muscle (Cat. # AKL99)