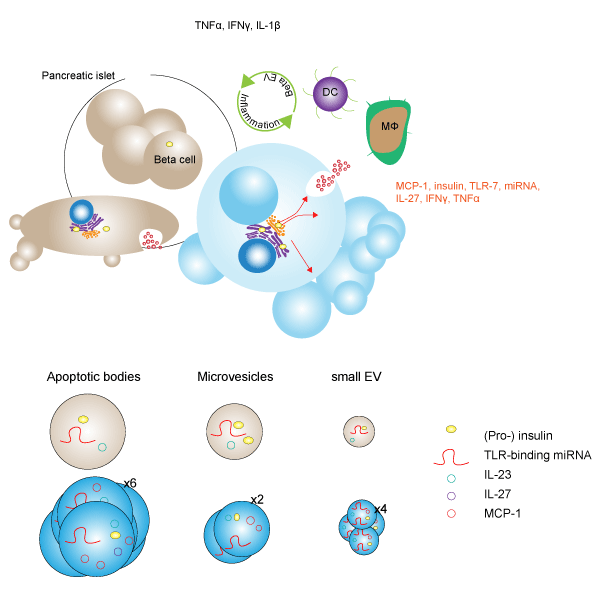
Pro-inflammatory stressors alter the vesiculome of MIN6 pancreatic beta cells
- By Cytoskeleton Inc. - Live Cell News
- Apr 13, 2021

The development of autoimmune type 1 diabetes is linked to chronic inflammation of pancreatic beta islet cells. While smaller extracellular vesicles (EVs), such as exosomes, have been implicated as key contributors to the development of pathogenic-inflammation in beta cells, the contribution of larger EVs such as apoptotic bodies (ABs), microvesicles (MVs), and small EVs (sEVs) is unknown. In a recent study, Giri et al. treated murine MIN6 beta cells with pro-inflammatory stressors such as incubation in 1% O2 (HX), ultraviolet irradiation (UV), and exposure to pro-inflammatory cytokines (CK), and characterized changes in the vesicular secretome, or vesiculome. Following treatment, investigators used size exclusion-based methods to isolate putative EVs. Lipid bilayer membrane analysis was performed with MemGlow in conjunction with cryo-electron microscopy to confirm EV capture. Examination of EVs with Tunable Resistive Pulse Sensing (TRPS) revealed that while stress-treatments of MIN6-derived EVs did not significantly change the size of individual vesicles, the number of EVs significantly increased ABs (5.5-fold), MVs (2.1-fold), and sEVs (4.5-fold) resulting in an overall increased vesiculome volume in a stress-dependent manner. CK-treatment resulted in the greatest vesiculome change at a 4.0-fold increase, with a 2.8-fold and 1.7-fold increase from UV- and HX-treatment, respectively. ELISA-quantitation of the islet cell autoantigen insulin revealed that while treatment with CK resulted in marked increases in total insulin levels in ABs and sEVs, the changes were due to an increase in vesicle number as the insulin content per EV particle did not change. Furthermore, insulin content of sEVs isolated from healthy cells showed a 4.6-fold lower content than ABs suggesting that insulin is predominantly carried in ABs and supports stress-induced increases in the insulin content of the vesiculome. Investigators also identified a significant drop in Toll Like Receptor 7 (TLR-7)-binding miRNAs in cells exposed to CK-treatment, which was concomitant with a 13- and 48-fold increase in large EVs and sEVs, respectively. Although blunted, UV- and HX-treatment recapitulated these findings in cells and large EVs, but not sEVs. This finding supports the idea that TLR-7-binding miRNAs are trafficked out of the cell in stress conditions predominantly through small EVs. To elucidate the impact of EV uptake on beta cells, investigators exposed primary NOD-mice bone marrow-derived dendritic (NOD-bmDC) cells to MIN6-derived beta EVs and found a significant upregulation of CD40 and major histocompatibility complex 2 (MHC II) arising from CK-treated ABs, but not MVs or sEVs. Treatment of bmDCs with sEVs and ABs derived from CK-treated MIN6 cells led to elevated TNFα revealing knock-on potential of pathologic EV-uptake. Altogether these data suggest that beta cell EV content is influenced by external pro-inflammatory signal in a stress-dependent manner. This study was enhanced by the utility of MemGlow probes to localize and confirm EV lipid bilayer membranes.
Note: The authors cite the use of MemBright Cy 3 and Cy 5 in their article. The US version of these dyes is MemGlow™ 560 and MemGlow™ 640, respectively.
Stress-induced alterations of the MIN6 pancreatic beta cell vesiculome
Link to Citation:
Related Products / Products Used: