Tubulin polymerization assay using >99% pure tubulin, OD based - Porcine (BK006P)
Product Uses Include
- Screening compounds for effects on tubulin polymerization activity.
- Screening proteins for effects on tubulin polymerization activity.
- Determining that tubulin alone (not MAPs) is the target for compounds that affect microtubule polymerization.
- Teaching aid for undergraduate/graduate class in pharmacology.
Introduction
This assay is based on an adaptation of the original method of Shelanski et al. and Lee et al. (1,2), which demonstrated that light is scattered by microtubules to an extent that is proportional to the concentration of microtubule polymer. The resulting polymerization curve is representative of the three phases of microtubule polymerization, namely nucleation, growth and steady state equilibrium. See the About Tubulin page for more information. The assay is optimized for a 96-well format for low CVs and efficient sample handling.
This kit contains the porcine neuronal tubulin of the highest available purity (>99% pure, Cat. # T240). The same type of assay is also available with HTS tubulin (Cat. # BK004P) and can serve as an economical, but slightly less sensitive alternative to BK006P. HTS tubulin (Cat. # HTS03) is >97% pure. Cytoskeleton, Inc. also provides a fluorescence based tubulin polymerization assay in miniaturized format (Cat. # BK011P) making it ideal for high throughput screening.
If you are interested in using either of these tubulin polymerization assays in a high throughput setting, please contact our technical service department for advice and bulk quotes.
Kit contents
This kit contains enough materials for 24 assays (BK006P). The following reagents are included:
- Tubulin, >99% pure, lyophilized (Cat. # T240)
- GTP solution (Cat. # BST06)
- General tubulin buffer (PEM, Cat. # BST01)
- Tubulin glycerol buffer (Cat. # BST05)
- Paclitaxel positive control (Cat. # TXD01)
- DMSO for paclitaxel.
- Half area 96-well plate for polymerization reactions
- Manual with detailed protocols and extensive troubleshooting guide.
Equipment needed
- 96-well plate spectrophotometer with filters to read optical density at 340 nm.
- Multi-channel pipette for rapid pipetting of tubulin
Example results
The BK006P kit was used to study the effects of Paclitaxel, a polymerization enhancer and Nocodazole, a polymerization inhibitor on tubulin polymerization (Fig. 1)
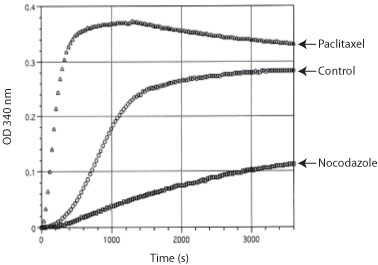
Figure 1. Tubulin polymerization curves from kit BK006P. The figure shows a standard polymerization curve (Control curve) containing a 100 µl volume of 3 mg/ml tubulin in 80 mM PIPES pH 7.0, 0.5 mM EGTA, 2 mM MgCl2, 1 mM GTP and 10% glycerol. Polymerization was started by incubation at 37°C and followed by absorption readings at 340 nm. Under these conditions, polymerization Vmax is enhanced 4 fold in the presence of 10 µM paclitaxel and reduced 5.5 fold in the presence of 10 µM nocodazole.
References
- Shelanski, M. L., Gaskin, F. and Cantor, C. R. (1973). Microtubule assembly in the absence of added nucleotides. Proc. Natl. Acad. Sci. U.S.A. 70, 765-768.
- Lee, J. C. and Timasheff, S. N. (1977). In vitro reconstitution of calf brain microtubules: effects of solution variable. Biochemistry, 16, 1754-1762.
For product Datasheets and MSDSs please click on the PDF links below. For additional information, click on the FAQs tab above or contact our Technical Support department at tservice@cytoskeleton.com
 For our Tubulin Polymerization (Absorbance) Excel Template please download here.
For our Tubulin Polymerization (Absorbance) Excel Template please download here. For our IC50 from Vmax Polymerization Excel Template please download here.
For our IC50 from Vmax Polymerization Excel Template please download here.
Question 1: What is the difference between this kit and BK004P?
Answer 1: Both the BK004P and BK006P are tubulin polymerization kits that are absorbance-based rather than fluorescence-based. The only difference between the two absorbance-based kits is that BK004P uses 97% pure tubulin (remaining 3% are MAPs) while BK006P uses >99% pure tubulin. This is an important difference because the presence of MAPs means that tubulin polymerization can be examined in the absence of enhancers such as glycerol or taxol with as little as 3 or 4 mg/ml tubulin using the BK004P kit. In this case MAPs act as polymerization enhancers. With BK006P, an enhancer such as glycerol or taxol must be used to drive tubulin polymerization with concentrations <5 mg/ml tubulin. Using tubulin at 5 mg/ml or higher allows for the omission of glycerol or taxol. In some cases, glycerol can interfere with the binding of tubulin accessory proteins or compounds. Assay conditions can easily be altered to test for glycerol interference.
Question 2: Which kit is best for screening a compound/reagent/drµg for its effects on tubulin polymerization?
Answer 2: All 3 tubulin polymerization kits (2 absorbance-based kits, BK004P and BK006P; 1 fluorescence-based kit, BK011P) are well-suited for screening of potential tubulin polymerization enhancers and inhibitors. Each kit has its own pros and cons. For initial compound/drµg screening, we recommend the absorbance-based tubulin polymerization assay BK004P. This kit uses 97% pure tubulin (remaining 3% are MAPs) while BK006P and BK011P use >99% pure tubulin. This is an important difference because the presence of MAPs means that tubulin polymerization can be examined in the absence of enhancers or inhibitors with as little as 3 or 4 mg/ml tubulin using the BK004P kit. To study enhancers, we recommend using 3 mg/ml tubulin, whereas 4 mg/ml tubulin is recommended for inhibitors. In the case of BK004P, MAPs act as polymerization enhancers. With BK006P and BK011P, an enhancer such as glycerol or taxol must be used to drive tubulin polymerization with concentrations <5 mg/ml tubulin. Using tubulin at 5 mg/ml or higher allows for the omission of glycerol or taxol, but requires additional tubulin. In some cases, glycerol can interfere with the binding of tubulin accessory proteins or compounds/reagents/drµgs. However, since BK011P is fluorescence-based, there is increased sensitivity that allows the researcher to use 1/3 as much tubulin with greater sensitivity. Thus, the kit provides 96 assays versus the 30 assays of BK004P or BK006P, thus BK011P is the most economical when requiring >30 assays for the project.
Question 3: How does porcine tubulin compare to bovine tubulin?
Answer 3: Click here for an in-depth comparison of porcine and bovine tubulin.
If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com




