Eg5 homolog kinesin motor domain protein (KIF11): GST tagged: Aspergillus fumigatis recombinant
Product uses
- Measurement of microtubule-activated ATPase activity
- Identification/characterization of proteins or small molecules that affect motor ATPase activity
- Identification/characterization of proteins or small molecules that affect kinesin motility
- Identification/characterization of proteins or small molecules that affect motor/microtubule interactions
Material
The conserved motor domain of Aspergillus fumigatus BimC kinesin (EG02) has been produced and purified from a prokaryotic expression system. The recombinant protein contains six histidine residues at the amino terminus (His-tag)and has an approximate molecular weight of 50 kDa. A. fumigatus BimC has been determined to be biologically active in a microtubule-activated ATPase activity test. The protein is supplied as a white lyophilized powder.
Purity
Protein purity is determined by scanning densitometry of Coomassie stained protein on a 12% gel. His-BimC protein was determined to be >95% pure (see Figure 1).

Figure 1. His-BimC Kinesin Motor Domain protein purity gel. A 10 µg sample of recombinant His-BimC protein (approx. 50 kDa) was separated by electrophoresis in a 12% SDS-PAGE system and stained with Coomassie blue. Protein quantitation was determined using Advanced Protein assay (Cat. # ADV02). Mark12 molecular weight markers are from Invitrogen.
Biological Activity - Microtubule Activated ATPase Assay
BimC ATPase activity was measured by monitoring real time free phosphate generation using the Kinesin ELIPA Assay Kit (Cat. # BK060). The assay is based upon an absorbance shift (330 nm to 360 nm) that occurs when 2-amino-6-mercapto-7-methylpurine ribonucleoside (MESG) is catalytically converted to 2-amino-6mercapto-7-methylpurine in the presence of inorganic phosphate (Pi). One molecule of Pi will yield one molecule of 2-amino-6mercapto-7-methylpurine in an essentially irreversible reaction. Hence, the absorbance at 360 nm is directly proportional to the amount of Pi generated in the kinesin ATPase reaction. Under the conditions outlined below, the Vmax for BimC microtubuleactivated ATPase activity has a minimum activity of 250 nmoles ATP generated per minute per mg of protein (Figure 2).
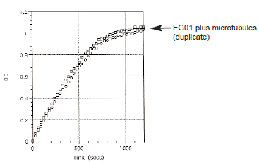
Figure 2. EG02 microtubule-activated ATPase activity. Recombinant BimC from A. fumigatus was assayed for microtubuleactivated ATPase activity in triplicate along with human recombinant Eg5 (Cat. # EG01) according to the method described. Control reactions were carried out in the absence of motor protein (microtubules only) and in the absence of microtubules (data not shown).
For product Datasheets and MSDSs please click on the PDF links below. For additional information, click on the FAQs tab above or contact our Technical Support department at tservice@cytoskeleton.com
Coming soon! If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com
