KIF11—also known as kinesin-5 or Eg5—is an essential mitotic motor protein that drives spindle pole separation and bipolar spindle assembly. Several small molecule inhibitors targeting KIF11 have been explored as anti-mitotic cancer therapies, though clinical efficacy as monotherapies has been limited, with filanesib showing promise in multiple myeloma.
The wild-type human motor domain of KIF11 is produced in a bacterial expression system.
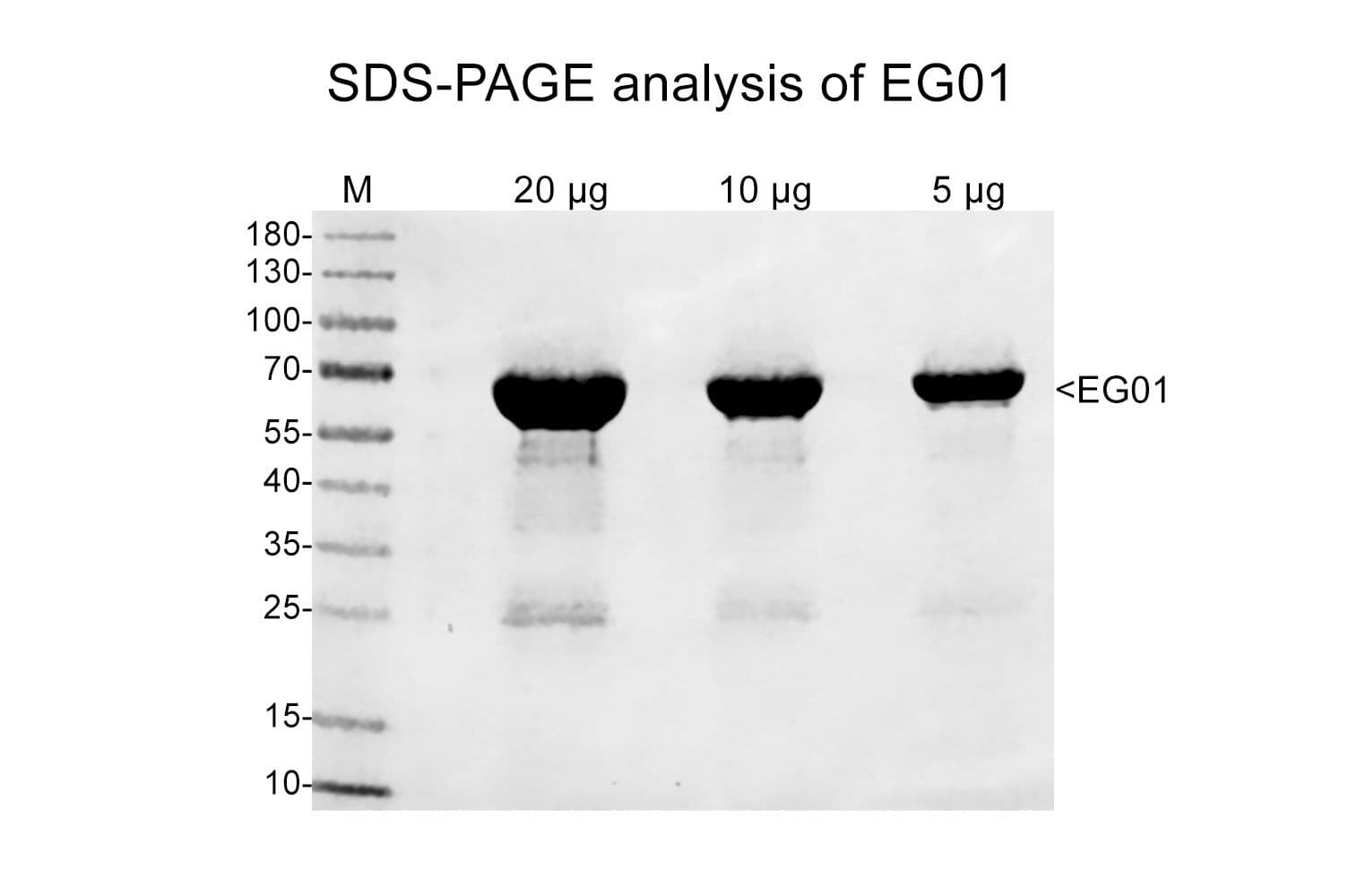
Protein purity is assessed by scanning densitometry of Coomassie Blue-stained protein on a4-20% polyacrylamide gel. Purity is determined to be >85% pure.
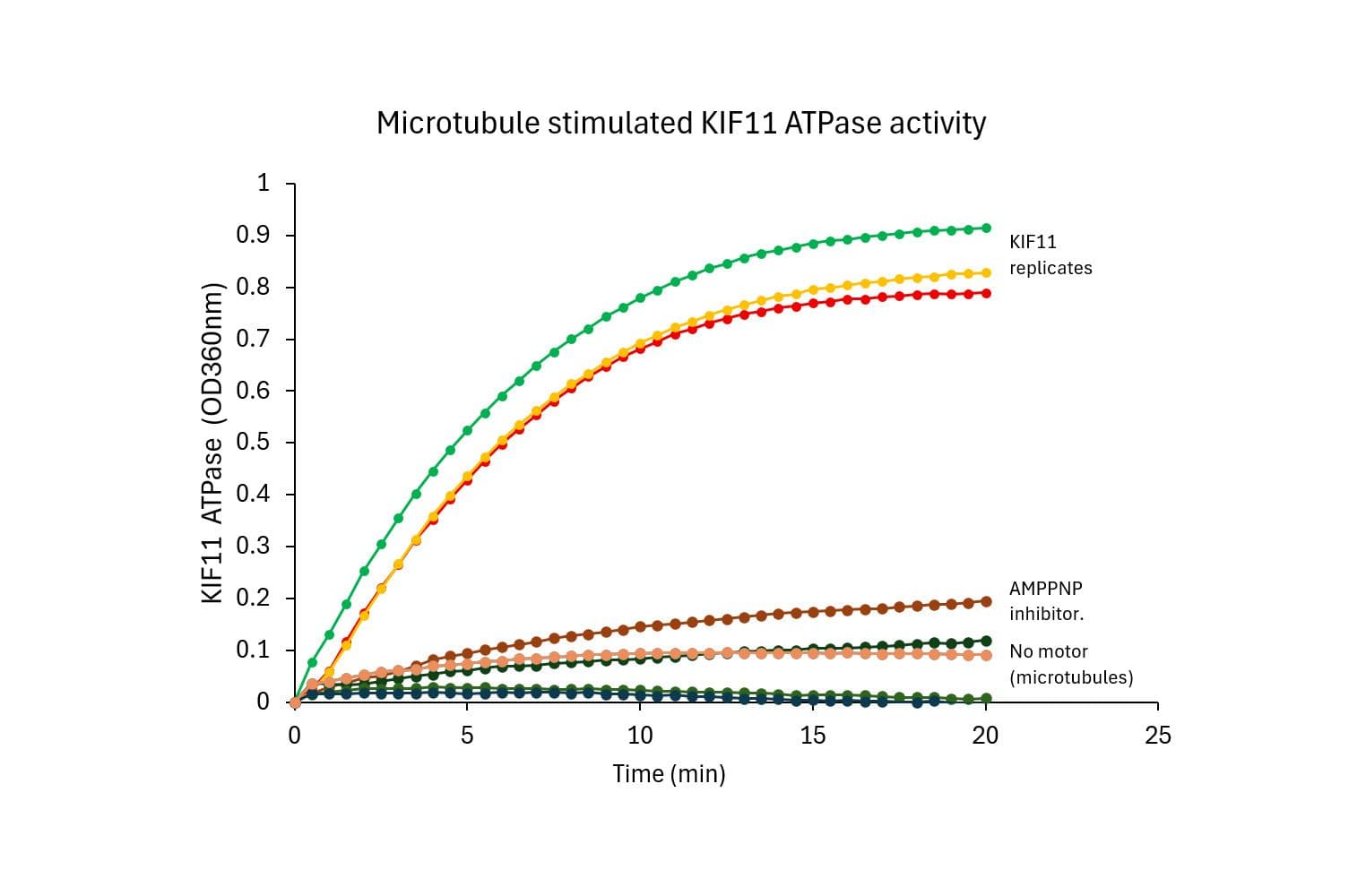
Biological activity of CS-KF11 is measured using a microtubule activated ATPase assay. Under the conditions stated (see datasheet), KIF11 exhibits a microtubule-stimulated ATPase activity with a Vmax of ≥ 600 nmol of ATP produced per minute per mg of KIF11.
Cat. #EG01