RalA G-LISA Activation Assay Kit (Colorimetric Based) 96 assays
The proprietary RalA G-LISA™ Activation Assay that is faster, easier and more precise than traditional pull-down methods to measure RalA Activation.
- Specific for RalA
- Colorimetric Based
- Fast Results
- Linear from 0.5 to 5 ng
- Flexible format
General Information
The RalA G-LISA™ kit contains a Ral-GTP-binding protein linked to the wells of a 96 well plate. Active GTP-bound Ral is captured by this protein while inactive GDP-bound Ral is removed following washes. The active RalA bound to the wells is detected with a RalA specific antibody. The degree of RalA activation is determined by comparing readings from activated cell lysates versus non-activated cell lysates.
Kit Contents - Enough reagents for 96 assays.
- 96 individual Ral-GTP binding wells
- Anti-RalA monoclonal antibody
- Secondary Antibody - HRP
- RalA control protein
- Ral Binding Buffer
- Cell Lysis Buffer
- Wash buffer
- Antigen Presenting Buffer
- Antibody Dilution Buffer
- HRP Detection and Stop Reagents
- Precision Red™Advanced Protein Assay (Cat. # ADV02)
- Protease Inhibitor Cocktail (Cat. # PIC02)
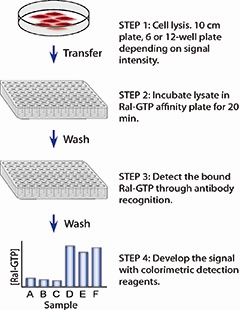
Figure 1.The simple procedure of the RalA G-LISA™ assay
Assay Linearity
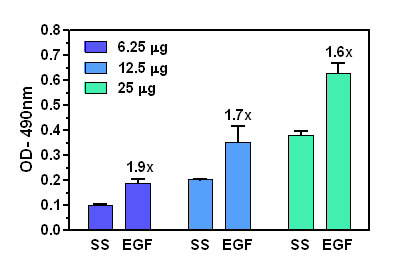
Figure 1: RalA activation by EGF measured by G-LISA™. Rat-2 cells were serum starved (SS) for 24 h and treated with EGF (100 ng/ml for 2 min). Lysates from these cells were tested at 25 μg/well, 12.5 μg/well and 12.5 μg/well starting concentrations in the RalA G-LISA™ assay. Absorbance was measured at 490 nm.
Maximal Ral activation window
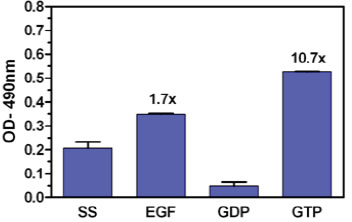
Figure 2: RalA activation measured using the Ral G-LISA™ format. Serum starved Rat-2 cell extract and EGF activated Rat-2 cell extract were incubated in separate wells of the G-LISA™ plate. Extracts from Rat-2 cells were also loaded with either GDP or GTP to investigate the maximal RalA activation window available in Rat-2 cells. Absorbance was read at 490 nm.
For product Datasheets and MSDSs please click on the PDF links below.
 G-LISA Activation Assay Technical Guide download here
G-LISA Activation Assay Technical Guide download here G-LISA Data Analysis (Absorbance) Excel Template download here.
G-LISA Data Analysis (Absorbance) Excel Template download here.
Question 1: Can I detect isoforms other than RhoA, Rac1 or RalA with these G-LISA activation assays?
Answer 1: Yes, the RhoA G-LISA (Cat. # BK124), Rac1 G-LISA (Cat. # BK128) and RalA G-LISA (Cat. # BK129) can be used to detect RhoB or RhoC, Rac 2 or Rac3 or RalB, respectively. The capture proteins that the wells have been coated with bind all of the isoforms of the respective GTPase. The specificity of signal is conferred by the specificity of the monoclonal primary antibody utilized. Use of an isoform-specific monoclonal antibody allows detection of other Rho family isoforms. Please see this citation for an example of this modified procedure (Hall et al., 2008. Type I Collagen Receptor (α2β1) Signaling Promotes Prostate Cancer Invasion through RhoC GTPase. Neoplasia. 10, 797–803).
Basically the researcher would test their specific monoclonal antibody in a western blot first to prove specificity to the alternative isoform of interest. For example, load RhoA and C for negative controls when testing a RhoB monoclonal antibody. Then the researcher would use 1:50, 1:200 and 1:500 dilutions of their monoclonal antibody on duplicate cell extracts of activated and control state samples. The researcher would then choose the dilution of monoclonal antibody which gave them the highest ratio of activated:control state.
A simple activated/control state pair of extracts can be made by growing cells to 50% confluence in serum containing media, washing twice with PBS, preparing lysate and aliquoting and freezing samples in liquid nitrogen. With one aliquot, defrost and let stand at room temperature for 60 min to degrade the activated signal to a low basal signal, which will be the control state. The untreated sample (2nd aliquot) will be considered “activated” which most serum grown cells are.
Question 2: How many cell culture plates can I process at one time during the lysis step?
Answer 2: We recommend that from the point at you add lysis buffer to the plate on ice to aliquoting and snap-freezing the lysate samples in liquid nitrogen, no more than 10 min are allowed to elapse. After 10 min on ice, we find that GTP bound to GTPases (activated GTPases) undergoes rapid hydrolysis. Rapid processing at 4°C is essential for accurate and reproducible results. The following guidelines are useful for rapid lysis of cells.
Washing
a. Retrieve culture dish from incubator, immediately aspirate out all of the media and place firmly on ice.
b. Immediately rinse cells with an appropriate volume of ice cold PBS (for Cdc42 activation, skip this step and simply aspirate the media) to remove serum proteins.
c. Aspirate off all residual PBS buffer. This is essential so that the Lysis Buffer is not diluted. Correct aspiration requires that the culture dish is placed at a steep angle on ice for 1 min to allow excess PBS to collect in the vessel for complete removal. As noted, the time period between cell lysis and addition of lysates to the wells is critically important. Take the following precautions:
1. Work quickly.
2. Keeping solutions and lysates embedded in ice so that the temperature is below 4°C. This helps to minimize changes in signal over time.
3. We strongly recommend that cell lysates be immediately frozen after harvest and clarification. A sample of at least 20 μl should be kept on ice for protein concentration measurement. The lysates must be snap frozen in liquid nitrogen and stored at -70°C. Lysates should be stored at -70°C for no longer than 30 days.
4. Thawing of cell lysates prior to use in the G-LISA assay should be in a room temperature water bath, followed by rapid transfer to ice and immediate use in the assay.
If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com.





