Citation Spotlight: Acetylated Microtubule Bundling, MAP Binding, and Functional Regulation of Kinesin-1
- By Cytoskeleton Inc. - Signal-Seeker News
- Jan 3, 2018

Balabanian et al. recently investigated the functional regulation of kinesin-1 motility using intact microtubules (MTs) in the form of either a single filament or bundles that were isolated from living COS-7 cells. Post-translational modifications (PTMs), MT bundling, and binding of microtubule-associated proteins (MAPs) are influential modulators of MT stability and motor protein activity. Here, the authors investigated the effects of the acetylation PTM, MT bundling, and binding of the MAP tau on kinesin-1 motility. Cytoskeleton’s SiR-tubulin live cell imaging probe and paclitaxel (Cat. # CY-SC002 and TXD01, respectively) were essential reagents in this kinesin-1 motility study, providing the tools necessary to examine the MT network in living cells and then confirm that it was maintained upon detergent extraction and isolation, followed by the necessary paclitaxel stabilization. The in vitro paclitaxel-stabilized, isolated MT single filaments and bundles faithfully recapitulated important structural attributes of an intact MT network in living cells. This in vitro model enabled the study of how kinesin-1 is functionally regulated by MT architecture, MAP binding, and PTMs such as acetylation. In sum, this model system provides a greater understanding of the physiological regulation of kinesin motors and kinesin-regulated transport of cargoes along MTs.
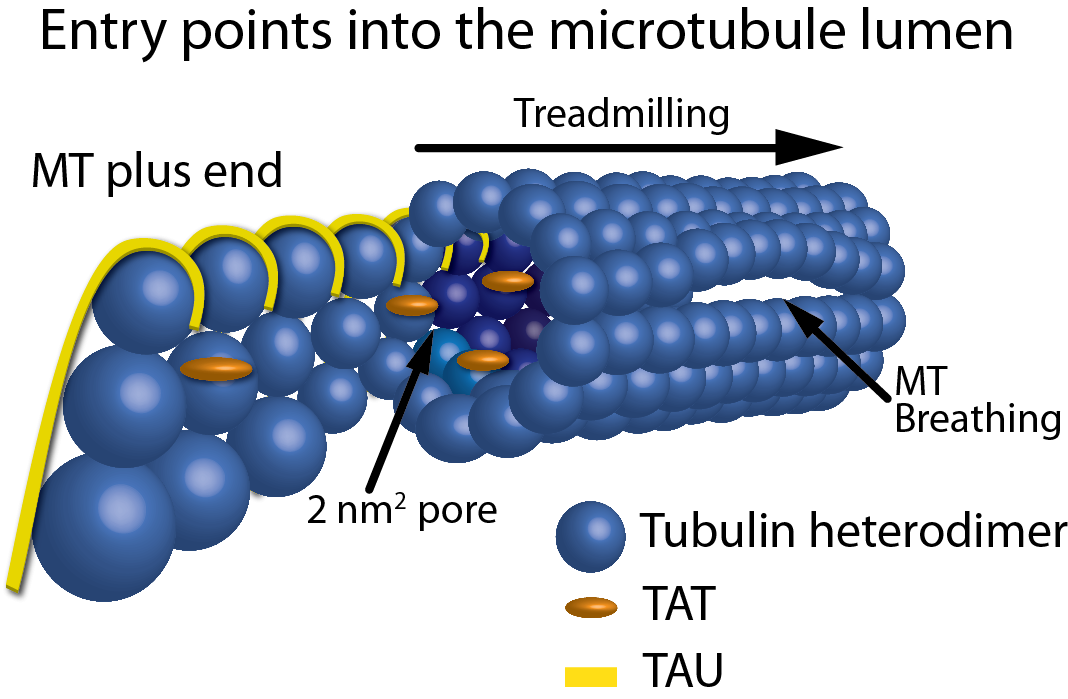
Figure 1: Schematic representation of the entry points into the MT lumen. Showing, from left to right, a frayed/growing MT plus end capturing TAT and tau molecules, treadmilling, 2 nm2 pores, a 200 nm2 open MT plus end, and a breathing MT lattice.
