Flipper-TR®: A Revolutionary New Fluorescent Probe for Measuring Membrane Tension in Cells and Tissues
Flipper-TR® is a live cell fluorescent membrane tension probe which breaks down the technical barriers hitherto circumvented by technical feats known only to biophysicists with custom equipment. The Flipper-TR membrane tension probe simplifies the methodology by using standard fluorescence lifetime measurements (see Flipper-TR FAQ for practical details). Here we describe the background of Flipper-TR in more detail.
Lipid membranes are dynamic, fluid structures (~4 nm thick) which is a biological necessity as they must change shape and tension for a cell to engage in basic cellular and subcellular physiological functions such as migration, cell spreading, phagocytosis, cell division, endocytosis, mechano-transduction, and metabolism, to name but a few. Consequently, membrane tension is under constant regulation due to its required dynamicity, and in turn, membrane tension regulates cell growth, development, motility, endocytosis, and metabolism. As the membrane is remodeled during these cellular processes, bending, tearing, and stretching of the membrane is common. These changes in membrane shape and tension occur over time and in different locations around and inside the cell and are important parameters to measure in order to understand how membrane tension is regulated and how it regulates these various basic, essential cellular processes. Understanding how membrane tension regulates cellular physiology is relevant in the study of healthy and diseased cells.
Membrane tension measurements usually relied on low resolution and slow physical methods to determine forces and tension within the plasma membrane. For example, a standard technique for measuring membrane tension involves pulling on membrane tubes from the plasma membrane with a bead trapped in an optical tweezer – a technique fraught with several methodological and technical limitations. For these reasons, novel, sensitive, reliable, and non-invasive research tools capable of rapidly measuring in vivo changes in membrane tension in real-time are in great demand. The fluorescent membrane tension probe Flipper-TR® (Spirochrome, Ltd.) answers these challenges as it has achieved unparalleled membrane tension sensitivity and temporal resolution through the use of FLIM (fluorescence lifetime imaging microscopy) to visualize Flipper-TR® staining of membranes in living cells. Flipper-TR® is a live cell fluorescent membrane tension probe and the first fluorescent membrane tension reporter developed for the field of mechanobiology. The fluorescence lifetime of Flipper-TR® is strongly dependent on the membrane tension. Using FLIM, the precise measurement of the spatio-temporal distribution of tension in membranes is now possible. Flipper-TR® opens up a whole new field by providing a sensitive, reliable, and non-invasive means by which rapid, real-time membrane tension in live cells is measured (Figure 1).
Figure 1 - Flipper-TR sensing membrane tension in cell undergoing hyperosmotic shock.
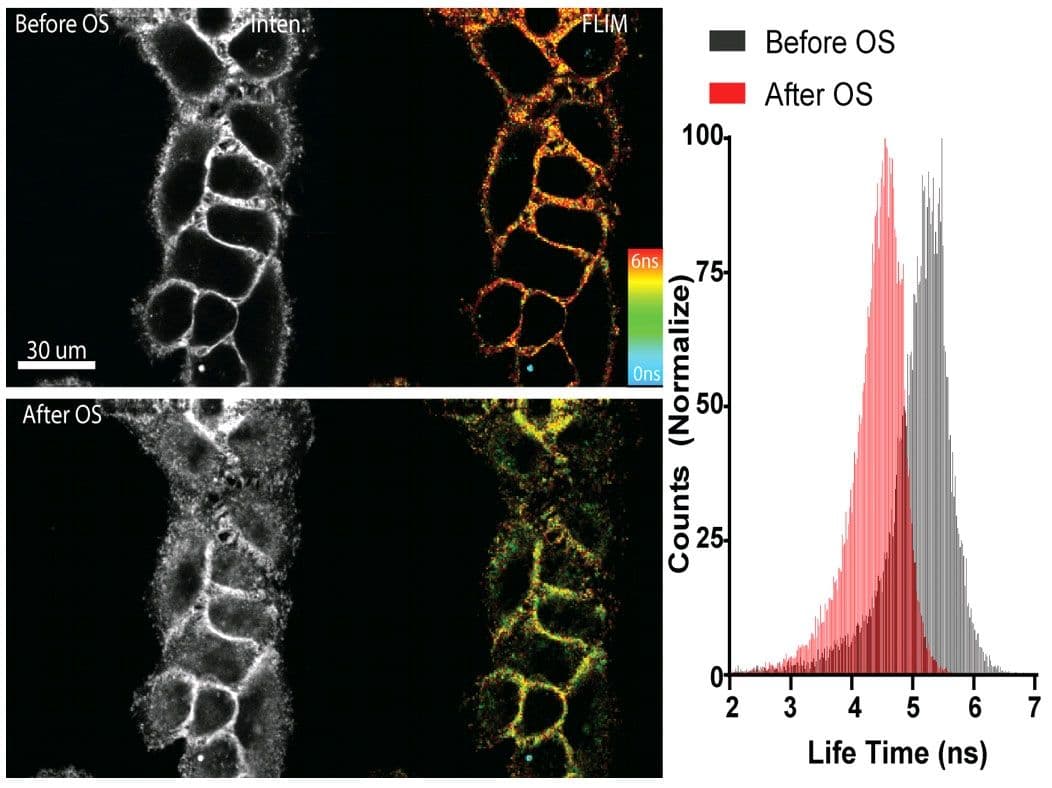
Legend Figure 1: Flipper-TR® staining of cells. Left side: Image of cells stained with Flipper-TR® before (top) and after (bottom) hyperosmotic shock. Greyscale represents fluorescence intensity, and color codes represent fluorescence lifetime. On the right the histogram shows the lifetime shift after osmotic shock. Images courtesy of Colom et al. 2018 (Ref. 1). Flipper-TR is a registered trademark of UNIGEM, Switzerland.
The fluorescent Flipper-TR® probe works by specifically targeting the plasma membrane of cells and reports membrane tension changes through its fluorescence lifetime changes. It is the most advanced member of the Flipper probes family2,3, which sense changes in the organization of lipid bilayer membranes through changes of the twist angle and polarization between the two twisted dithienothiophenes of the mechanophore. Flipper-TR® spontaneously inserts into the plasma membrane of cells and is only fluorescent when inserted into a lipid membrane. It has a broad absorption and emission spectrum; excitation can be commonly performed with a 488 nm laser, while emission is collected between 575 and 625 nm (Figure 2).
Figure 2 - Absorbance and emission spectra of Flipper-TR
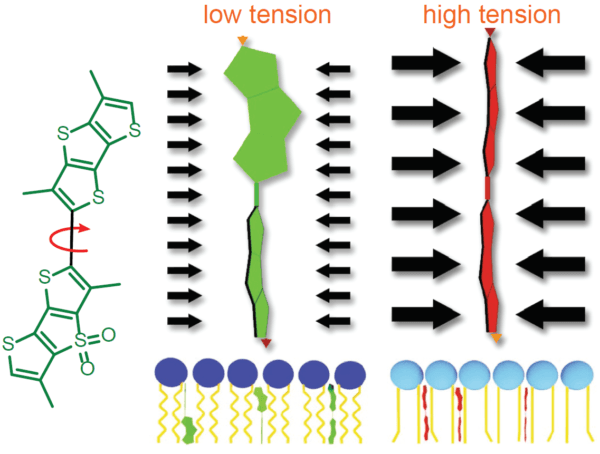
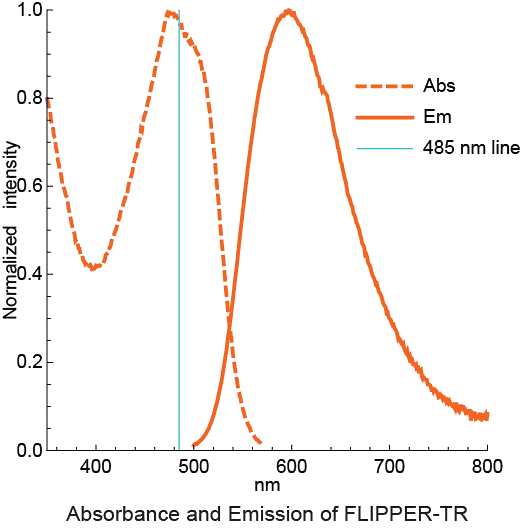
Legend Figure 2 - A. Schematic diagram of the mechanism of Flipper-TR and its interaction with membranes with different tension. On the left, basic molecular structure of Flipper-TR. On the right, low tension green twisted Flipper-TR and on the right high tension planar structure. B. Flipper-TR solution in ethyl acetate was subject to absornace and emission scans and the results plotted on the same graph. Absorbance is dotted orange line, and emission is a solid orange line.
A detailed protocol for staining and FLIM imaging can be found below the References (click here, and Ref.5). Flipper-TR® works on a wide range of organisms including bacteria, mammalians, plants, and yeast1,4 (Figure 3).
Figure 3 - Flipper-TR staining in yeast cells
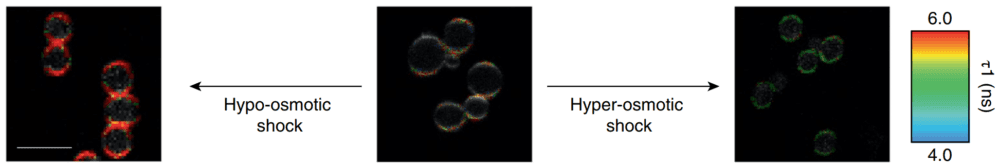
Legend for Figure 3: Flipper-TR® staining in yeast cells. Yeast cells stained with Flipper-TR and treated with either hypo-osmotic choc (left) or hyper-osmotic choc (right). The fluorescence lifetime of Flipper-TR shifts to high or low values depending on the applied conditions. The color codes for fluorescence lifetime. Images courtesy of M. Riggi from Roux group at UNIGE (Ref. 4). Note the large pseudo-color change which is proprotional to the tension in the membrane.
Data analysis
The photon histograms from ROI or single pixels are fitted with a double-exponential, and two decay times (τ1 and τ2) are extracted. The longest lifetime with the higher fit amplitude τ1 is used to report membrane tension and varies between 2.8 and 7.0 ns. Longer lifetime means more tension in the membrane. τ2 with a smaller value (between 0.5 and 2 ns) and a small fit amplitude is less suited to study membrane tension. The lifetime can be correlated to absolute membrane tension using the calibration procedure given in Ref. 1 (Colom et al. 2018).
Figure 4 - Schematic diagram of short and long lifetime photon release.
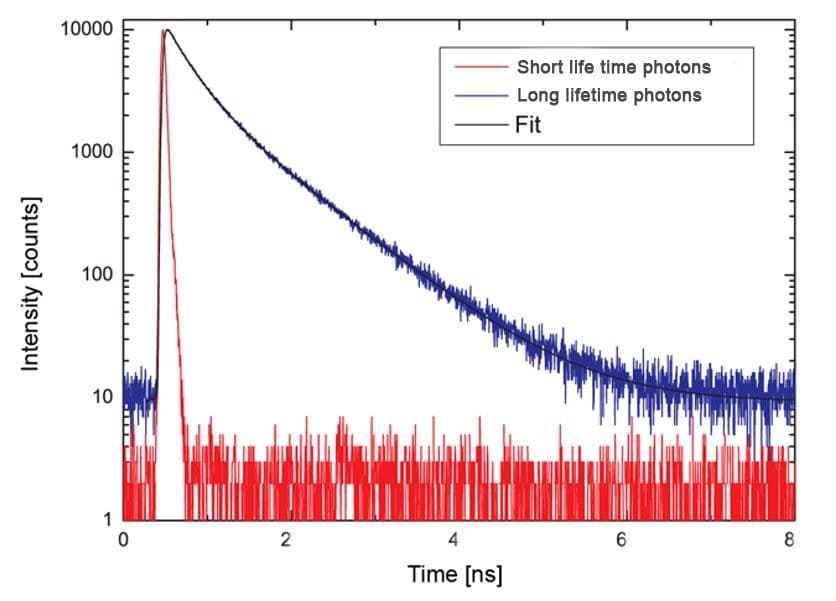
Legend Figure 4 - Short lifetime photons represented by the red dashes. Long lifetime photons represented by the blue dashes. Note - these data are not derived from Flipper-TR but represents the effect of two types of fluorescent lifetime decay.
References
- Colom A et al. 2018. A fluorescent membrane tension probe. Nat. Chem. 10, 1118–1125.
- Dal Molin M. et al. 2015. Fluorescent flippers for mechanosensitive membrane probes. J. Am. Chem. Soc. 137, 568-571.
- Soleimanpour S. et al. 2016. Headgroup engineering in mechanosensitive membrane probes. Chem. Commun. (Camb). 52, 14450-14453.
- Riggi M et al. 2018. Decrease in plasma membrane tension triggers PtdIns(4,5)P2 phase separation to inactivate TORC2. Nat. Cell Biol. 20, 1043–1051.
- FLIM microscopy: Lakowicz JR et al. 1994. Emerging biomedical and advanced applications of time-resolved fluorescence spectroscopy. J Fluoresc. 4(1):117-36. doi: 10.1007/BF01876666.
Protocol 1: General Labeling Protocol (consult the datasheet and published papers for detailed protocols)
- Grow cells on coverslips, glass-bottom dishes, or glass-bottom multi-well plates based on standard laboratory cell culture protocols. When cells have reached the desired density, reconstitute Flipper-TR. Optimal labeling conditions for each cell type should be empirically determined.
- Reconstitute and prepare a 1 mM master stock solution of Flipper-TR. Store as directed on the datasheet.
- Prepare a working solution from the 1 mM master stock for staining of the cultured cells. Start with 1 μM stain in cell culture medium. Replace the culture medium with the staining solution.
- Return the cells to the incubator at 37°C in a humidified atmosphere containing 5% CO2 for 15 minutes before imaging.
- Image stained cells with standard FLIM techniques using a 485 or 488 nm pulsed laser for excitation and collecting photons through a 600/50 nm bandpass filter. Optimization of the labeling procedure and image acquisition settings is recommended so that photodamage is minimized. NOTE: Membrane tension measurements can only be performed by FLIM (fluorescence intensity or wavelength are not reliable).
Data Analysis
For extraction of lifetime information, the photon histograms from ROI or single pixels are fitted with a double-exponential, and two decay times (τ1 and τ2) are extracted. The longest lifetime with the higher fit amplitude τ1 is used to report membrane tension and varies between 2.8 and 7.0 ns. Longer lifetime means more tension in the membrane. τ2 with a smaller value (between 0.5 and 2 ns) and a small fit amplitude is less suited to study membrane tension. The lifetime can be correlated to absolute membrane tension using the calibration procedure given in reference # 1 (Colom et al. 2018).