+3
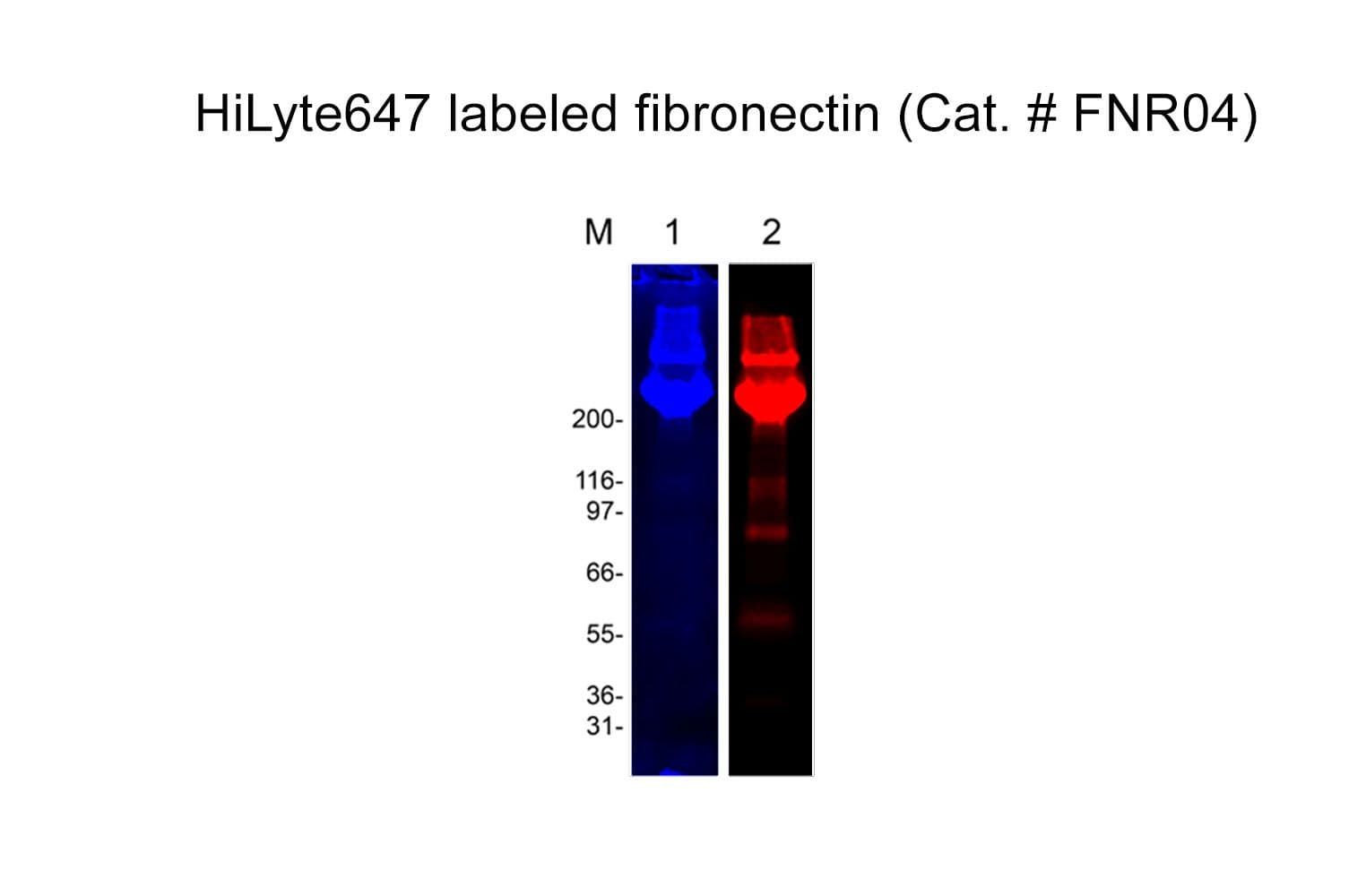
Fibronectin is purified from bovine plasma; it is a high-molecular-weight (~440kDa) glycoprotein, made up of two subunits that vary in size between 235-270 kDa (due to alternate splicing). The secreted fibronectin dimer is a soluble protein that polymerizes to higher-order fibrils in the extracellular matrix (ECM).
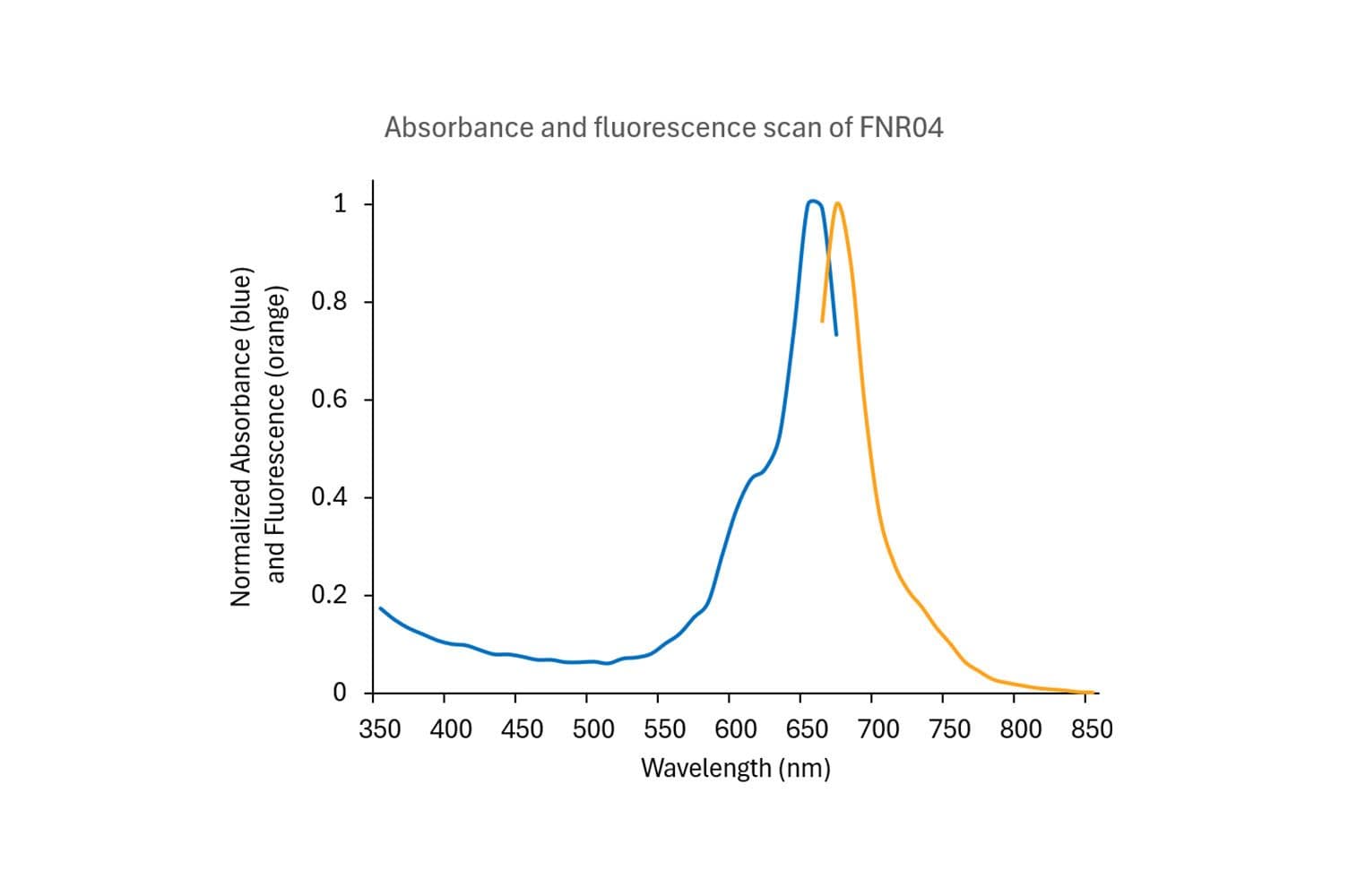
The protein is modified to contain covalently linked HiLyte™647 at random surface lysines.
Protein purity is determined by scanning densitometry of Coomassie Blue-stained protein on a 4-20% polyacrylamide gel. FNR04 is >80% pure.
Biological activity of FNR04 is demonstrated by its ability to act as a substrate for cell invasion, as monitored through an ECM degradation assay.
Cat. #FNR04