Repurposing Colchicine To Treat Covid-19
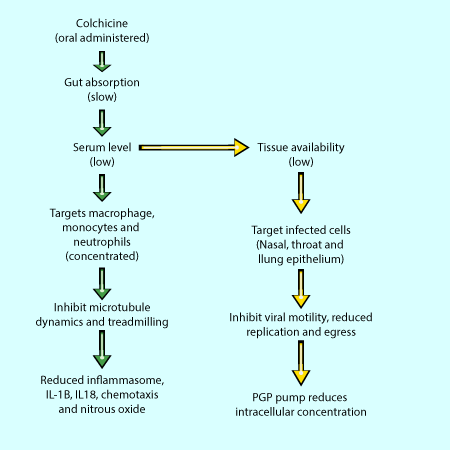
Above: Therapeutic pathways of colchicine through the body.
References
1. Kolle K, Martin MA, Antia R, Lopman B, and Dean NE. 2022. The changing epidemiology of SARS-CoV-2. Science, 375 (6585), p.1116-1121. DOI: 10.1126/science.abm4915
2. NIH webpage: https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/colchicine/
3.Ebers Papyrus, BC 1550. https://en.wikipedia.org/wiki/Ebers_Papyrus.
4. Schlesinger N, Firestein BL, Brunetti L. 2020. Colchicine in COVID-19: an Old Drug, New Use. Curr Pharmacol Rep, 6(4), p.137-145. doi: 10.1007/s40495-020-00225-6.
5. Montealegre-Gómez G, Garavito E, Gómez-López A, Rojas-Villarraga A, Parra-Medina R. 2021. Colchicine: A potential therapeutic tool against COVID-19. Experience of 5 patients. Reumatol Clin (Engl Ed), 17(7), p.371-375. doi: 10.1016/j.reumae.2020.05.008.
6. FDA webpage: https://clinicaltrials.gov/ct2/results?cond=covid-19&term=colchicine&cntry=&state=&city=&dist=
7. Borel JF, Feurer C. 1975. Chemotaxis of rabbit macrophages in vitro: inhibition by drugs. Experientia, 31(12). P.1437-9. doi: 10.1007/BF01923232. PMID: 814017
8. Paschke S, Weidner AF, Paust T, Marti O, Beil M, and Ben-Chetrit E. 2013. Technical advance: Inhibition of neutrophil chemotaxis by colchicine is modulated through viscoelastic properties of sub-cellular compartments. J. Leukoc. Biol. 94, p.1091-96.
9. Chia EW, Grainger R, and Harper JL. 2008. Colchicine suppresses neutrophil superoxide production in a murine model of gouty arthritis: a rationale for use of low dose colchicine. Br. J. Pharmacol. 153, 1288-95.
10. Martinon F, Petrilli V, Mayor A, Tardivel A, and Tschopp J. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature, 440(7081), p.237-241.
11. Fordham JN, Kirwan J, Cason J, and Currey HL. 1981. Prolonged reduction in polymorphonuclear adhesion following oral colchicine. Ann. Rheum. Dis. 40(6), p.605-608. Pubmed: 7332382.
12. Borisy GG, Taylor EW. 1967. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J. Cell Biol. 34(2):525-33. doi: 10.1083/jcb.34.2.525. PMID: 6068183
13. Downing K, Nogales E. 1998. Tubulin structure: insights into microtubule properties and functions. Current Opinion in Structural Biology, 8(6), p. 785-791. ISSN 0959-440X. doi.org/10.1016/S0959-440X(98)80099-7.
14. Amandine O. 2019. Tubulin-VDAC Interaction: Molecular Basis for Mitochondrial Dysfunction in Chemotherapy-Induced Peripheral Neuropathy. Frontiers in Physiology, 10, article 671, p.1-8. DOI:10.3389/fphys.2019.00671.
15. Shi L. and Tu BP. 2015. Acetyl-CoA and the Regulation of Metabolism: Mechanisms and Consequences. Curr Opin Cell Biol. 33, p. 125–131. doi:10.1016/j.ceb.2015.02.003.
16. Glebov OO. 2020. Understanding SARS-CoV-2 endocytosis for Covid-19 drug repurposing. FEBS Jo. 287, p. 3664-3671. Doi:10.1111/febs.15369.
17. Wen Z, Zhang Y, Lin Z, Shi K, Jiu Y. 2020. Cytoskeleton-a crucial key in host cell for coronavirus infection. J Mol Cell Biol. 12(12), p.968-979. doi: 10.1093/jmcb/mjaa042.
18. Kloc M, Uosef A, Wosik J, Kubiak JZ, Ghobrial RM. 2022. Virus interactions with the actin cytoskeleton-what we know and do not know about SARS-CoV-2. Arch Virol. 167(3), p.737-749. doi: 10.1007/s00705-022-05366-1.
19. Norris V, Ovádi J. 2021. Role of Multifunctional Cytoskeletal Filaments in Coronaviridae Infections: Therapeutic Opportunities for COVID-19 in a Nutshell. Cells. 10(7), p.1818. doi: 10.3390/cells10071818.
20. Oliva MÁ, Tosat-Bitrián C, Barrado-Gil L, Bonato F, Galindo I, Garaigorta U, Álvarez-Bernad B, París-Ogáyar R, Lucena-Agell D, Giménez-Abián JF, García-Dorival I, Urquiza J, Gastaminza P, Díaz JF, Palomo V, Alonso C. 2022. Effect of Clinically Used Microtubule Targeting Drugs on Viral Infection and Transport Function. Int J Mol Sci. 23(7), p.3448. doi: 10.3390/ijms23073448.
21. Chappey O, Niel E, Dervichian M, Wautier JL, Scherrmann JM, Cattan D. 1994. Colchicine concentration in leukocytes of patients with familial Mediterranean fever. Br J Clin Pharmacol. 38(1), p.87-9. doi: 10.1111/j.1365-2125.1994.tb04328.x.
22. Margolis RL, Wilson L. 1977. Addition of colchicine-tubulin complex to microtubule ends: the mechanism of substoichiometric colchicine poisoning. Proc Natl Acad Sci U S A. 74(8), p.3466-70. doi: 10.1073/pnas.74.8.3466.
23. Maekawa S. Dissociation constant of Tetrahymena tubulin-colchicine complex. J Biochem. 1978 Sep;84(3):641-6. doi: 10.1093/oxfordjournals.jbchem.a132169. PMID: 721798.
24. Bergen LG, and Borisy GG. 1983. Tubulin-colchicine complex inhibits microtubule elongation at both plus and minus ends. J Biol Chem. 258(7), p.4190-4. PMID: 6833250.
25. Skoufias DA, Wilson L. Mechanism of inhibition of microtubule polymerization by colchicine: inhibitory potencies of unliganded colchicine and tubulin-colchicine complexes. Biochemistry. 1992 Jan 28;31(3):738-46. doi: 10.1021/bi00118a015.
26. Vandecandelaere A, Martin SR, Schilstra MJ, Bayley PM. Effects of the tubulin-colchicine complex on microtubule dynamic instability. Biochemistry. 1994 Mar 15;33(10):2792-801. doi: 10.1021/bi00176a007.
27. Batchuluun B, Pinkosky SL, & Steinberg GR. 2022. Lipogenesis inhibitors: therapeutic opportunities and challenges. Nat Rev Drug Discov 21, 283–305. https://doi.org/10.1038/s41573-021-00367-2.
28. Tardif J-C, Bouabdallaoui N, L'Allier PL, Gaudet D, Shah B, Pillinger MH, et al. 2021. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. The Lancet, 9(8), p.924-932. DOI:https://doi.org/10.1016/S2213-2600(21)00222-8 .
29. Bouhaddou M, Memon D, Meyer B, White KM, Rezelj VV, et al. 2020. The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell, 182(3), p.:685-712.e19. doi: 10.1016/j.cell.2020.06.034.
30. Bojkova, D., Klann, K., Koch, B. et al. 2020. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature, 583, p.469–472. https://doi.org/10.1038/s41586-020-2332-7.
31. Stukalov, A., Girault, V., Grass, V. et al. 2021. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature, 594, 246–252. https://doi.org/10.1038/s41586-021-03493-4.
32. Hekman RM, Hume AJ, Goel RK, Abo KM, Huang J, et al. 2020. Actionable Cytopathogenic Host Responses of Human Alveolar Type 2 Cells to SARS-CoV-2. Mol Cell. 80(6), p.1104-1122.e9. doi: 10.1016/j.molcel.2020.11.028.
33. Veru website: https://verupharma.com/pipeline/sabizabulin-for-covid-19/ .
Related Products:
Pre-formed Microtubules
Purified Tubulins and MAPS
Tubulin protein (97% pure): porcine brain (Cat. # HTS03)
Tubulin Kits
Tubulin polymerization HTS assay using >97% pure tubulin, OD based - Porcine (Cat. # BK004P)
Tubulin polymerization assay using >99% pure tubulin, OD based - Porcine (Cat. # BK006P)
Tubulin polymerization assay using >99% pure tubulin, fluorescence based (Cat. # BK011P)
Microtubule Binding Protein Spin-Down Assay Biochem Kit (Cat. # BK029)
Microtubule/Tubulin In Vivo Assay Biochem Kit (Cat. # BK038)
Tubulin Live Cell Imaging Tools
SiR-Tubulin Kit (Cat. # CY-SC002)
Cytoskeleton Kit (Includes SiR-Actin, SiR-Tubulin, and Verapamil) (Cat. # CY-SC006)
SiR700-Tubulin Kit (Cat. # CY-SC014)

