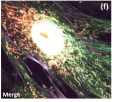
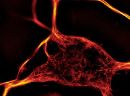
Mito Flipper-TR Kit for fluorescence cell membrane microscopy
Mito Flipper-TR Kit, a probe for measuring plasma-membrane tension.
Mito Flipper-TR® is a live cell fluorescent probe that specifically targets the Mitochondrial membrane of cells and reports membrane tension changes through its fluorescence lifetime changes. It is one of the targeted Flipper probes family(1-2) which sense changes of the organization of lipid bilayer membranes through changes of the twist angle and polarization between the two twisted dithienothiophenes of the mechanophore. Mito Flipper-TR® spontaneously localizes to the mitochondrial membrane of cells and is only fluorescent when inserted in the lipid membrane. It has a broad absorption and emission spectrum, excitation can be commonly performed with a 488nm laser, while emission is collected between 575 and 625nm
Mito Flipper-TR® use requires Fluorescence Liftime Imaging Microscopy (FLIM) which is a standard technique in modern microscopic set-ups, for more details see FAQ tab.
For Mito Flipper-TR® datasheet click here.
Optical properties
λabs 480 nm
λem 600 nm
εmax 1.6x104 mol-1·cm-1
For more detailed information see the About tab.
Mito Flipper-TR is a registered trademark of UNIGEM, Switzerland. Cytoskeleton, Inc. is the exclusive provider of Spirochrome, Ltd. products in North America and non-exclusive Worldwide distributor.
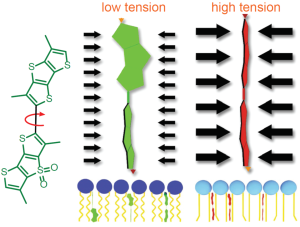
Schematic representation of the mechanism of action of Flipper-TR tension reporting properties
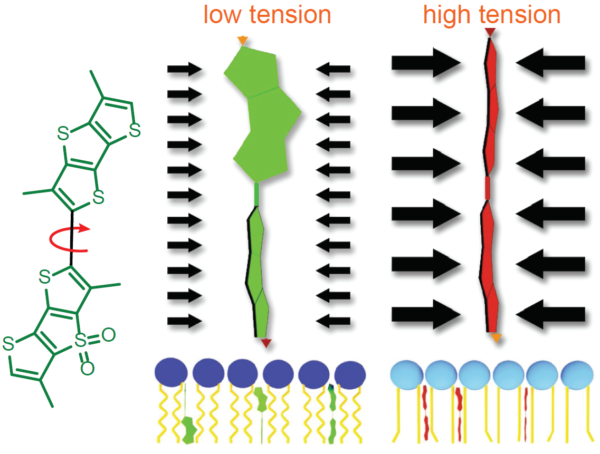
Figure legend: On the left; schematic image of the Flipper-TR molecule. In the middle a low tension lipid bilayer with Flipper twisted, on the right the probe is planar.
Labelling Protocol
Note: This protocol was optimized using HeLa cells adhering to coverslips and has been confirmed in other common cell lines. Recommendations for experimental protocols should be used as a starting point, and optimal labeling conditions for each cell type should be determined empirically.
Prepare 1 mM stock solution. Dissolve the content of the vial of Mito Flipper-TR® in 35 μL of anhydrous DMSO to make a 1 mM stock solution. This solution should be stored at -20°C or below. Do not divide the solution into small aliquots, they will decay faster and the compound is not altered by multiple freeze-thaw cycles. When stored properly, this stock solution is stable for up to three months. (Optional) If the concentration of the stock solution needs be accurately determined, dilute 1 µl of 1 mM stock solution in 99 µl of DMSO. Measure the absorbance at 425 nm. Calculate the concentration using the extinction coefficient given above.
Prepare staining solution. Dilute Mito Flipper-TR® DMSO stock solution to the desired concentration (start with 1 µM) in cell culture medium shortly before applying to the cells (Apply quickly, max 5 minutes, the staining solution to the cells of which the growth medium was removed). Note: when using a cell culture media supplemented with Fetal Calf Serum (FCS) or other serum proteins, the efficiency of labelling will be reduced compared to media devoid of serum. If a low signal is observed, the probe concentration can be increased up to 2-3 µM.
Cell preparation and staining.
Grow cells on coverslips, glass bottom dish or glass bottom multi-well plates as usual. When cells have reached the desired density, replace the culture medium by the staining solution (prepared shortly before) ensuring that all the cells are covered with solution. Place the cells in the incubator at 37°C in a humidified atmosphere containing 5% CO2 low tension high tension for 15 minutes before imaging. Optionally, the medium containing the probe can be removed, and cells washed once in fresh growth media. As the probe is fluorescent only in membranes, the probe does not need to be removed, especially in cases where the staining medium contains serum when long term imaging (>24h) is planned. No impact on cell viability has been observed on HeLa cells at concentrations up to 5 µM.
FLIM imaging.
Cells are imaged with standard FLIM microscopes using a 485 or 488 nm pulsed laser for excitation and collecting photons through a 600/50 nm bandpass filter. We recommend optimizing the labeling procedure as well as the image acquisition settings to minimize photodamage induced by the 488nm excitation light on live samples. To extract lifetime information, the photon histograms from ROI or single pixels (accumulate sufficient counts to ensure good statistics) are fitted with a doubleexponential, and two decay times, τ1 and τ2 are extracted. The longest lifetime with the higher fit amplitude τ1 is used to report membrane tension and varies between 2.8 and 7.0 ns. Longer lifetime means more tension in the membrane. τ2 with a smaller value (between 0.5 and 2 ns) and a small fit amplitude is less suited to study membrane tension. The lifetime can be correlated to absolute membrane tension using the calibration procedure given in Reference 1. In HeLa cells, average 1 lifetimes in mitochondrial membranes were around 3.2 ns while 3.3 ns in COS7 cells. Hyperosmotic shock (0.5 M sucrose) lowers lifetimes by ~0.2 ns.3)
Important notes:
- Membrane tension measurements can only be performed by FLIM microscopy, fluorescence intensity or wavelength is not reliably reporting on membrane tension.
- Systems where the membrane lipid composition changes over time may also induce a change of Mito Flipper-TR® lifetime.
-FLIM imaging is an advanced microscopy technique requiring a commercial or custom built FLIM microscopy system with the adequate excitation lasers, photon counting systems and emission filters. Customers are advised to consult their instrument responsible person or contact the microscope manufacturer to ensure that their system is able to image Mito Flipper-TR® fluorescence and lifetime.
References:
1) Colom A, et al: A fluorescent membrane tension probe. Nat Chem, 2018, 10:1118–1125 ().
2) Dal Molin M, et al: Fluorescent flippers for mechanosensitive membrane probes. JACS, 2015, 137:568-571.
3) Goujon A, et al: Mechanosensitive Fluorescent Probes to Image Membrane Tension in Mitochondria, Endoplasmic Reticulum, and Lysosomes, J. Am. Chem. Soc. 141, 8, 3380–3384, 2019.
Mito Flipper-TR® probe is distributed by Spirochrome under an exclusive license from the University of Geneva, Switzerland and was developed by the NCCR Chemical Biology.
FAQs of the revolutionary Flipper-TR® probe
Q1. What is FLIM microscopy and how does it work for Flipper-TR?
A1. FLIM microscopy stands for Fluorescent Lifetime Imaging Microscopy. The importance for membrane tension studies is that prior to Flipper-TR membrane tension measurements were very labor and equipment intensive, but now relatively straightforward adaptation of your current microscope will enable highly sensitive tension measurements. Nowadays it is a very standard technique with equipment available from many suppliers, it is based on recording the time that emission takes after excitation of the fluorophore, which is usually very rapid, on the order of 1-10 nano-seconds. FLIM can also be combined with other high resolution microscopic techniques such as Total Internal Reflection Fluorescence (TIRF) or Stimulated Emission Depletion (STED) microscopy for high spatial resolution. FLIM microscopy requires time resolved light detectors which many scientific microscope vendors have available, for example PicoQuant's upgrade kit (https://www.picoquant.com/news/item/picoquants-flim-fcs-upgrade-kit-now-supports-zeiss-lsm780-and-leica-sp2). Reference 1 describes more details about the experimental setup for FLIM microscopy. (Flipper-TR is a registered trademark of UNIGEM, Switzerland)
Figure 1 - Diagram of FLIM set-up and time resolved color coded cell image.
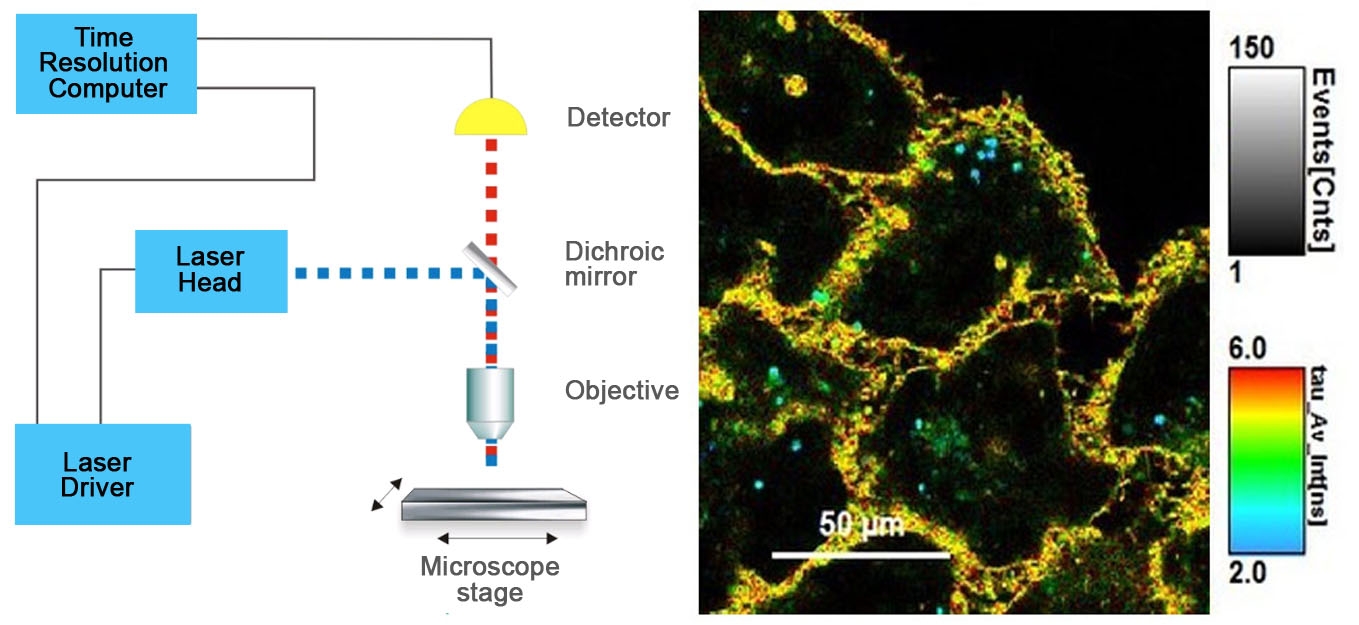
Figure 1. On the left, standard FLIM microscope layout arranged in simple units that are readily available from microscope vendors. On the right, image of cells stained with Flipper-TR®, greyscale represents fluorescence intensity, and color codes represent fluorescence lifetime. Images courtesy of Colom et al. 2018 (Ref. 1). Flipper-TR is a registered trademark of Spirochrome SA, Switzerland.
Q2. Does the Flipper-TR probe work to change fluorescence lifetime?
A2. The fluorescent Flipper-TR® probe works by specifically targeting the plasma membrane of cells and reports membrane tension changes through its fluorescence lifetime changes. It is the most advanced member of the Flipper probes family (Ref. 2,3,4,5). Flipper-TR® spontaneously inserts into the plasma membrane of cells and is only fluorescent when inserted into a lipid membrane. The probe senses changes in the organization of lipid bilayer membranes through the twist angle and polarization between two twisted dithienothiophenes of the mechanophore (see Fig. 2). The emission lifetime is short (2-4 ns) when in the tense state (dithienothiophenes aligned), and longer in the relaxed state (4.1-8.0 ns, dithienothiophenes twisted). Variance (cv) is in the order of 0.3 ns (cv = 4-15%) which allows high resolution of subtle changes in membrane tension. Typically the shorter lifetimes are color coded green, medium lifetimes coded yellow, and longer lifetimes are orange and red (see Fig. 2).
Figure 2 - Schematic diagram of the structure and mechanism of tension reporting by Flipper-TR

Legend Figure 2. Schematic diagram of the mechanism of Flipper-TR and its interaction with membranes with different tension. On the left, basic molecular structure of Flipper-TR. On the right, low tension green twisted Flipper-TR and on the right high tension planar structure.
Q3. What are the filter sets for these probes?
A3. The Flipper-TR probe is visualized with a long separation filter set because its excitation peak is more than 100 nm shorter than the emission peak. Thus the ideal filter set is an excitation of 488 +/- 20 nm and an emission of 575 to 675 +/- 40 nm (Fig. 3). The time resolved measurement method allows very low bacground, which is additive with its low fluorescence in aqueous envirnments, see Q4 below.
Figure 3 - Absortion and emission spectra of Flipper-TR
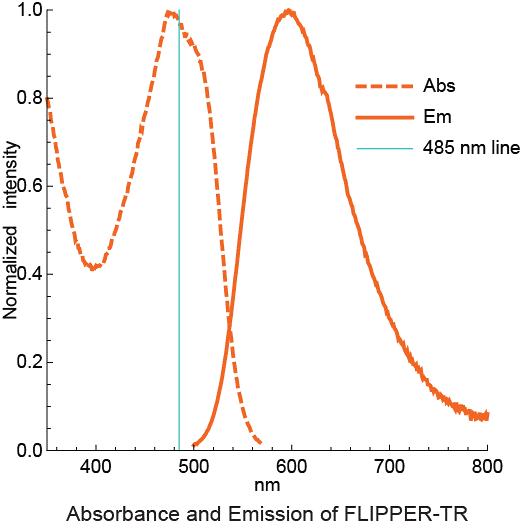
Legend Figure 3 - Flipper-TR solution in ethylacetate was subject to absornace and emission scans and the results plotted on the same graph. Absorbance is dotted orange line, and emission is a solid orange line.
Q4. Why does the Flipper-TR probe have a low background compared to other plasma-membrane probes?
A4. The Flipper-TR probes has very low background in aquence enviroments e.g. tissue culture media or fixative buffer, because it is a fully twisted state and tends to form micelles which quenches itself (Ref.3). After insertion into the membrane it becomes less twisted and starts to emit with high fluorescence (see Fig. 2).
Q5: Is the Flipper-TR probe stable at room temperature?
A5: Yes, the probe is stable in the powder form at room temperature for a few days. After reconstitution in anhydrous DMSO (do not use old-pre-opened bottles of DMSO, but do use ampoules of dry DMSO from Sigma or Spectrum Chemicals. It is stable to freezing and thawing at –20°C, but it is not recommended to divide into small aliquots for storage because it will degrade under these conditions.
Q6: Is the Flipper-TR toxic to cells?
A6: No, under the conditions given in the datasheet the probe is not toxic. Cells will be viable and fluorescent for 2-4 days depending on cell type and culture condition.
Q7: Which organisms and tissues are stained by the Flipper-TR probe?
A7: Currently all known organism have been stained with Flipper-TR, these include tissue culture cells, tissue section (fresh), mammalian cells, insect cells, plant cells, yeast and bacteria.
Q8. Does the Flipper-TR probe work in 3D cell cultures?
A8: Yes, the probe is able to stain cells in a 3D growth environment.
Q9: What is the quantum yield and extinction coefficient in the membrane?
A9: Quantum yield = 0.30 in ethylacetate.
References
1. FLIM microscopy: Lakowicz JR et al. 1994. Emerging biomedical and advanced applications of time-resolved fluorescence spectroscopy. J Fluoresc. 4(1):117-36. doi: 10.1007/BF01876666.
2. Riggi M et al. 2018. Decrease in plasma membrane tension triggers PtdIns(4,5)P2 phase separation to inactivate TORC2. Nat. Cell Biol. 20, 1043–1051.
3. Colom A et al. 2018. A fluorescent membrane tension probe. Nat. Chem. 10, 1118–1125.
4. Dal Molin M. et al. 2015. Fluorescent flippers for mechanosensitive membrane probes. J. Am. Chem. Soc. 137, 568-571.
5. Soleimanpour S. et al. 2016. Headgroup engineering in mechanosensitive membrane probes. Chem. Commun. (Camb). 52, 14450-14453.