Citation Spotlight: Actin Bundling Protein EFhd2 Mediates LPS-Induced Macrophage Migration
- By Cytoskeleton Inc. - Actin News
- Mar 6, 2018

Tu et al. recently characterized the functional relationship between the novel actin bundling protein EFhd2 and actin during lipopolysaccharide (LPS)-induced macrophage migration, an important component of innate immune responses. The authors found that LPS-mediated macrophage migration depends upon EFhd2 and its regulation of actin polymerization and subsequent re-organization of the actin cytoskeleton. EFhd2 and F-actin co-localization in macrophages is essential for LPS’s actions and coincides with a LPS-induced increase in the ratio of F-actin to G-actin in these cells, an effect prevented by knock-out of EFhd2. Under cell-free conditions, EFhd2 increases actin polymerization in a concentration-dependent manner and to a magnitude similar to that induced by the nucleation factors Arp2/3 + VCA domain of the Wiskott-Aldrich syndrome protein (WASP). This suggests that EFhd2’s effects are through a direct regulation of the actin cytoskeleton. Cytoskeleton’s actin polymerization assay kit, G-actin/F-actin in vivo assay kit, Acti-stain 555 phalloidin, Arp2/3 protein complex, and VCA domain of WASP (Cat. # BK003, BK037, PHDH1, RP01P, and VCG03, respectively) were essential reagents in this characterization study. These kits and reagents provided the tools necessary to examine how EFhd2 and its regulation of actin polymerization and cytoskeletal dynamics participate in LPS-induced macrophage motility.
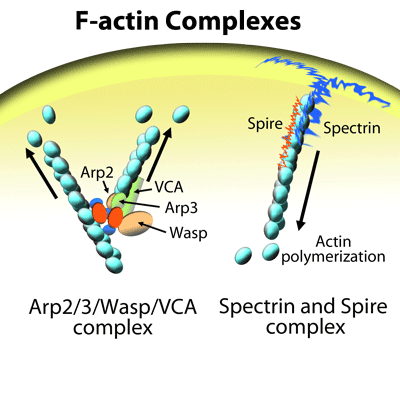
Nucleation of actin filaments (F-actin) mediated by a complex consisting of the actin nucleation factors Arp2, Arp3, and WASP (VCA domain).
