Neuropathy-causing TRPV4 mutations disrupt TRPV4-RhoA interactions and impair neurite
- By Cytoskeleton Inc. - Small G-Protein News
- Sep 12, 2022

Neuropathy-causing TRPV4 mutations disrupt TRPV4-RhoA interactions and impair neurite extension
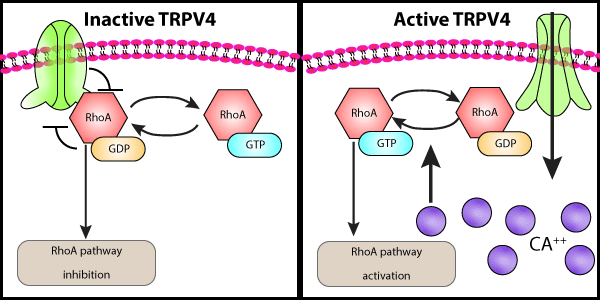
Transient receptor potential vanilloid 4 (TRPV4) is a homotetrameric ion channel that coordinates a variety of cellular processes in response to extracellular environmental stimuli. Interestingly, mutations in TRPV4 result in an array of pathologies such as neuropathy and skeletal dysplasia; nonetheless, the cause of these diseases appears to have a common underlying factor, that is, a dysfunction in cytoskeletal processes. Recently, McCray et al. sought to understand the mechanisms by which TRPV4-mutations alter cytoskeletal function and neuropathy. Utilizing an unbiased proteomic approach they identified RhoA as a novel binding partner. Utilizing purified proteins, the group showed that the N-terminal ankyrin repeat domain (ARD) of TRPV4 specifically interacts with GDP-bound RhoA. TRPV4 neuropathy-specific mutations, which cluster in the ARD region of TRPV4, significantly disrupted the interaction between TRPV4 and RhoA binding. The binding of RhoA to TRPV4 disrupted pharmacologic activation of TRPV4; conversely, TRPV4 can act like a RhoGDI inhibiting protein and prevent bound RhoA from becoming activated. However, the group determined that under specific stimulus the TRPV4 channel can become activated, promote calcium influx, and release RhoA which then becomes activated. This dual ability to regulate RhoA was found to be critical in TRPV4’s ability to regulate cell morphology and affect neuropathy in both in vitro cell models and in vivo fly models of neuropathy. Cytoskeleton Inc’s Rho inhibitor, Exoenzyme C3 Transferase Protein (Cat. #CT03) was essential to study the role of RhoA on TRPV4-mediated neurite outgrowth. The interplay between TRPV4 and RhoA is complex, but this study uncovers a novel interaction between these proteins that may play an important role in TRPV4-mutant-dependent neuropathy.
Link to Citation:
Related Products/Products Used
Exoenzyme C3 Transferase Protein: His Tagged:Clostridium Botulinum Recombinant (Cat. # CT03)
Cdc42 Pull-Down Activation Assay Biochem Kit (Bead Pull-Down Format) - 50 Assays (Cat. # BK034)
Rac1 Pull-Down Activation Assay Biochem Kit (Bead Pull-Down Format) - 50 Assays (Cat. # BK035)
RhoA Pull-Down Activation Assay Biochem Kit (Bead Pull-Down Format) - 80 Assays (Cat. # BK036)