Porcine Hemagglutinating Encephalomyelitis Virus (PHEV) Orchestrates Cytoskeletal and Transcriptional Changes of Host Neurons to Support Invasion
- By Cytoskeleton Inc. - Actin News
- Apr 28, 2020

Porcine Hemagglutinating Encephalomyelitis Virus (PHEV) is an enveloped neurotropic single-stranded RNA coronavirus, belonging to the family Coronaviridae and genus Betacoronavirus, that causes profound neurological symptoms in pigs. PHEV infection entails the removal of key barriers to entry including the temporary dissolution or remodeling of protective cortical actin. In this study authors thoroughly interrogated the mechanism of PHEV infection on N2a cells and its subsequent cellular consequences. Investigators found PHEV infection was followed by actin cytoskeleton remodeling that included vacillating abundance of stress fibers, filopodia, and lamellipodia. Phosphorylation analysis of cofilin, a regulator of filamentous actin, revealed a link between actin remodeling and cofilin activation. Modulating actin remodeling by transfecting constitutively active, inactive, or wild-type cofilins resulted in attenuated PHEV penetration. Similarly, CytochalasinD-induced collapse of the cytoskeleton reduced viral titers and taken altogether suggests that functional actin and cytoskeletal regulation through biphasic cofilin activation is required for successful infection. When upstream factors of cofilin regulation were pharmacologically interrogated via ATN-161-induced inhibition of integrin α5β1, and PF-573228-induced inhibition of non-receptor tyrosine protein kinase FAK, viral entry was significantly inhibited concomitant with cofilin phosphorylation suggesting that PHEV binding to integrin α5β1 induces FAK-signaling that in turn promotes PHEV infiltration. Given the influence GTPases exert on actin dynamics the authors then inhibited Rac1 and Cdc42 with EHoP-016 and ML-141, respectively, which impacted cofilin phosphorylation during invasion and subsequently inhibited viral entry. Furthermore, a pulldown assay revealed that chronological activation of Rac1 and Cdc42 corresponded with cofilin phosphorylation and actin dynamics during infection, and this effect was reversible by ATN-161 and PF-573228. LIM Domain Kinase (LIMK) and p21-activated kinase PAK1, which reside downstream of Rac1 and Cdc42, were shown to be phosphorylated during early infection. When PAK1, which phosphorylates LIMK, was selectively inhibited via IPA-3 PHEV-induced formation of filopodia and lamellipodia, as well as cofilin phosphorylation and viral entry were inhibited. The authors conclude that PHEV infection initiates viral entry through an integrin α5β1-FAK-Rac1/Cdc42-PAK-LIMK signaling pathway that elicits cofilin-induced actin remodeling required for viral entry and trafficking. The majority of the interrogations of viral invasion found in this study were conducted with microscopical visualization and quantification, as well as flow cytometry quantification, and greatly enhanced by Cytoskeleton’s fluorescently conjugated phalloidin product Acti-stain 488™ (Cat.# PHDG1).
Note: The authors unintentionally refer to the labeled phalloidin as FITC-phalloidin; however, based on our product lineup we conclude that they are referring to Acti-stain 488™ which uses a proprietary dye distinct from FITC.
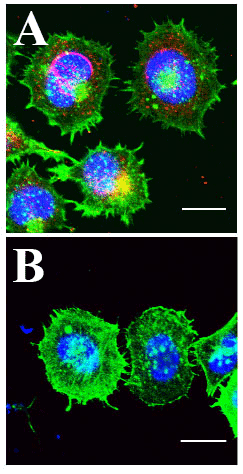
Figure 1 Legend:
A) HCT-8 Cells Infected with PHEV coronavirus. Actin labeled with Cytoskeleton Acti-stain 488 (green), PHEV coat protein antibody labeled (red) and DNA with Hoechst (blue). Cells exhibit high level of PHEV coat protein at the nuclear periphery, multiple lamellipodia and filopodia, and decreased cortical and cytoplasmic actin.
B) Uninfected HCT-8 control cells. Relative to infected cells, nuclear peripheral signal of PHEV coat protein is absent, appearance of lamellipodia and filopodia is reduced, and bright ubiquitous cortical and cytoplasmic actin is abundant. Kindly provided by Prof. Wenqi He, from Lv et al. 2019).
Products Used in this Citation:
Directly Related Products:
Acti-stain™ 535 (Cat. # PHDR1)
Acti-stain™ 555 (Cat. # PHDH1)
Acti-stain™ 670 (Cat. # PHDN1)
Cdc42 Pull-down Activation Assay Biochem Kit (bead pull-down format) - 50 Assays (Cat. # BK034)
Rac1 Pull-down Activation Assay Biochem Kit (bead pull-down format) - 50 Assays (Cat. # BK035)
Rac1 G-LISA Activation Assay (Luminescence format) - 96 assays (Cat. # BK126)
Cdc42 G-LISA Activation Assay (Colorimetric format) - 96 assays (Cat. # BK127)
Rac1 G-LISA Activation Assay Kit (Colorimetric Based) 96 assays (Cat. # BK128)