Amyloid beta-induced F-actin destabilization alters dendritic spines in models of Alzheimer’s disease
- By Cytoskeleton Inc. - Actin News
- Dec 8, 2020

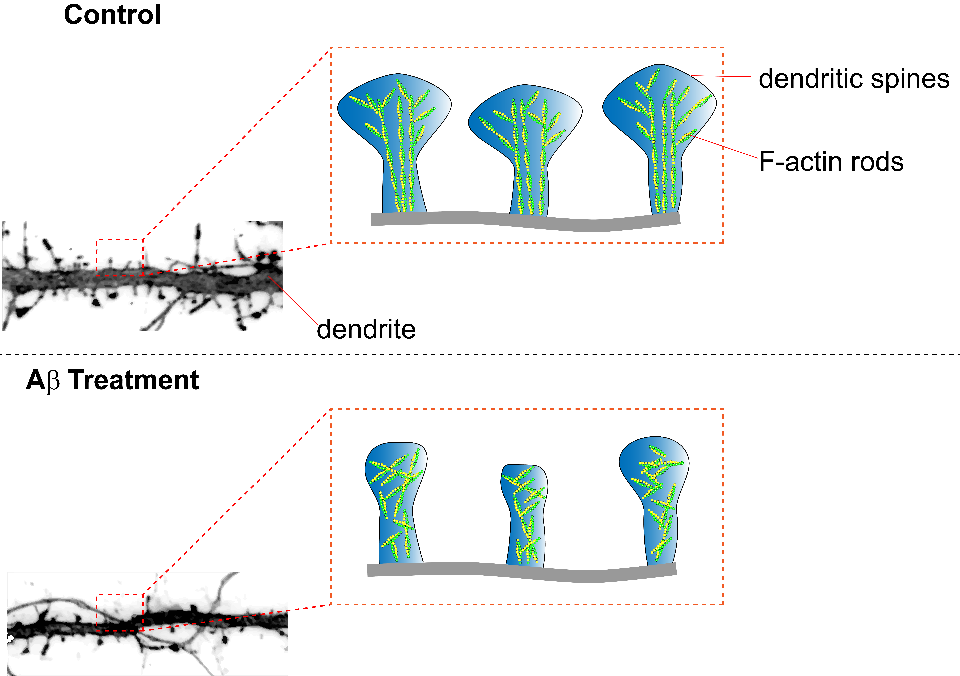
Alzheimer’s disease (AD) is a neurodegenerative disorder manifest by impaired cognitive function and memory loss. Various changes in neuron morphology underlie AD’s clinical symptoms including synapse loss; in mouse models of AD this is represented via a loss of dendritic spines. Dendritic spines are enriched with helical filamentous-actin (F-actin), a predominant cytoskeleton protein, and undergo remodeling for the stabilization of memories after learning. A study by Kommadi et al. investigated whether increased pools of G-actin and decreased F-actin preceded AD onset or were a consequence of dendritic spine loss. Relative to WT littermate controls, synaptosomes in mouse models of AD (APP/PS1) displayed diminished levels of F-actin and increased G-actin, despite not having a decrease in total actin nor displaying pathologic hallmarks of the disease (Cat. # BK037). Pyrene-actin assays (Cat. # BK003) revealed that actin in the presence of Aβ1-42 peptide displayed disrupted polymerization kinetics similar to APP/PS1 actin polymerization. Long-term in vitro staining of primary cortical neurons with DiI or phalloidin (Cat. # PHDG1) revealed that a reduction of F-actin was present at day 10 in APP/PS1 neurites without concomitant change in dendritic spine number or surface area. At day 16 a marked decrease of spines, spine dendrites, and spine surface area was observed in APP/PS1 neurons, concomitant with a significant reduction of F-actin in tertiary neurites. Primary cortical neurons stained with phalloidin and treated with Ab42 exhibited dose-dependent diminishment of F-actin, suggesting amyloid-β can depolymerize F-actin. APP/PS1 mice displayed a reduced fear conditional potential relative to WT controls, that was reversible with actin-stabilizing jasplakinolide, suggesting disruption of F-actin perturbs memory formation. Relative to wild-type neurons, APP/PS1 mushroom spine heads labeled with phalloidin or DiI were quantified with dSTORM and exhibited significantly reduced actin rod length and order arising from altered F-actin architecture. Examination of G:F actin ratios (Cat. # BK037) in post-mortem synaptosomes from humans with no cognitive impairment (NCI), mild cognitive impairment (MCI), and AD revealed significantly decreased F-actin without change in G-actin. A significant correlation was also found between reduced F-actin and diminished global cognition, β-amyloid levels, neurofibrillary tangles, and Braak staging. Cytoskeleton’s actin tools highlighted above played an essential role in revealing the importance that F-actin has in AD progression and it will be interesting to further unravel the interaction between actin and AD.
Caption 1:Summary figure illustrating the effect of amyloid beta treatment on neurons. Dendritic spines treated with amyloid beta peptides exhibit decreased dendritic spine surface area, F-actin rod length, and anisotropy (parallel arrangement of F-actin rods).
Products Used:
Actin Polymerization Biochem Kit (fluorescence format): rabbit skeletal muscle actin (Cat. # BK003)
G-Actin/F-actin In Vivo Assay Biochem Kit (Cat.# BK037)
Related Products:
Acti-stain™ 535 (Cat. # PHDR1)
Acti-stain™ 555 (Cat. # PHDH1)
Acti-stain™ 670 (Cat. # PHDN1)
MemGlow™ 488: Fluorogenic Membrane Probe (a member of the MEMBRIGHT™ family) (Cat. # MG01))
MemGlow™ 560: Fluorogenic Membrane Probe (a member of the MEMBRIGHT™ family) (Cat. # MG02)
MemGlow™ 590: Fluorogenic Membrane Probe (a member of the MEMBRIGHT™ family) (Cat. # MG03)
MemGlow™ 640: Fluorogenic Membrane Probe (a member of the MEMBRIGHT™ family) (Cat. # MG04)
MemGlow™ 700: Fluorogenic Membrane Probe (a member of the MEMBRIGHT™ family) (Cat. # MG05)
SPY555-actin (Cat. # CY-SC202)
SiR700-Actin Kit (Cat. # CY-SC013)
SiR-Actin Kit (Cat. # CY-SC001)
Anti-Pan Actin Mouse Monoclonal Antibody (Clone 7A8.2.1) (Cat. # AAN02)