+3
Kit contents (20-50 assays)
Equipment & materials required
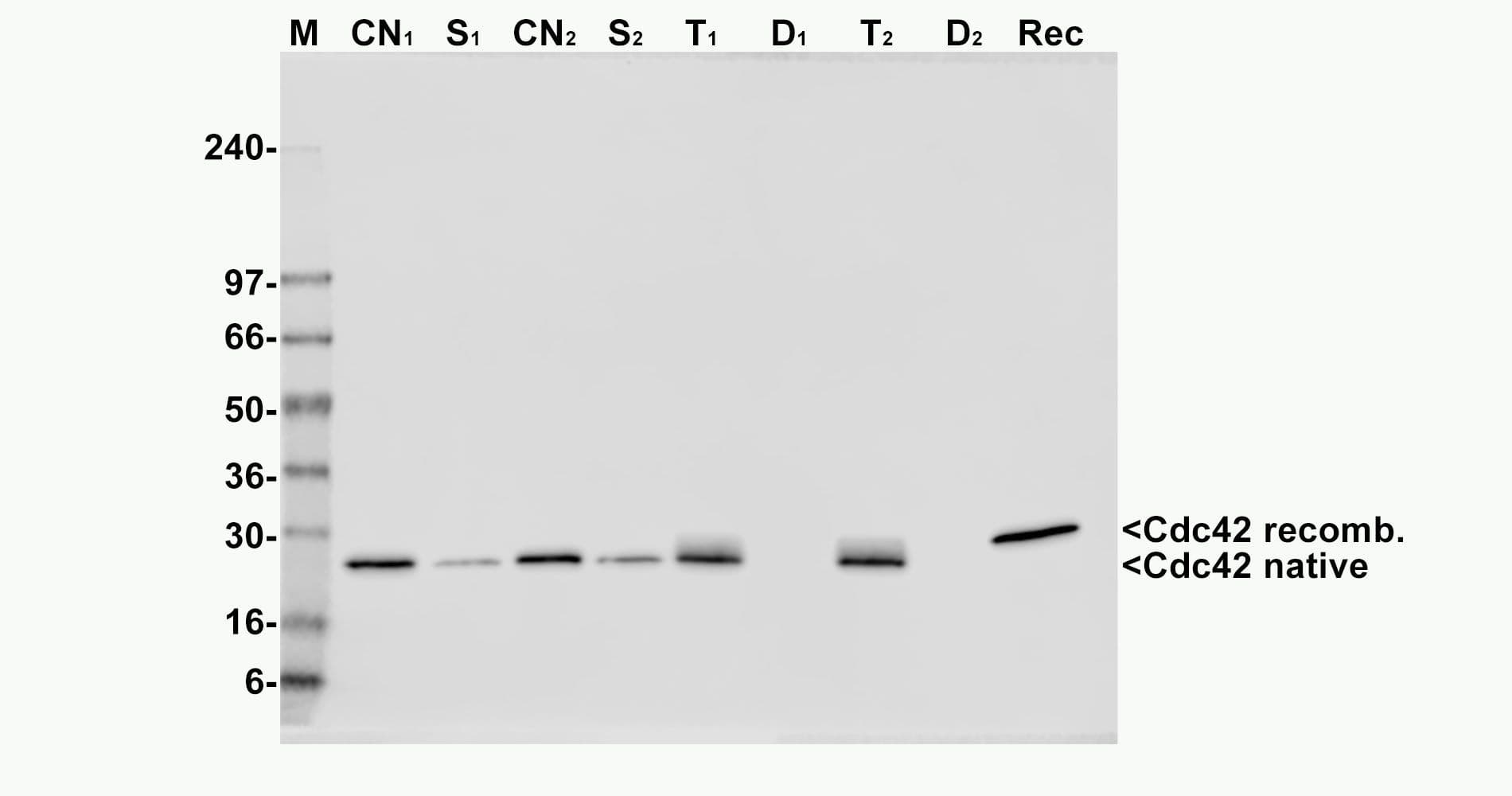
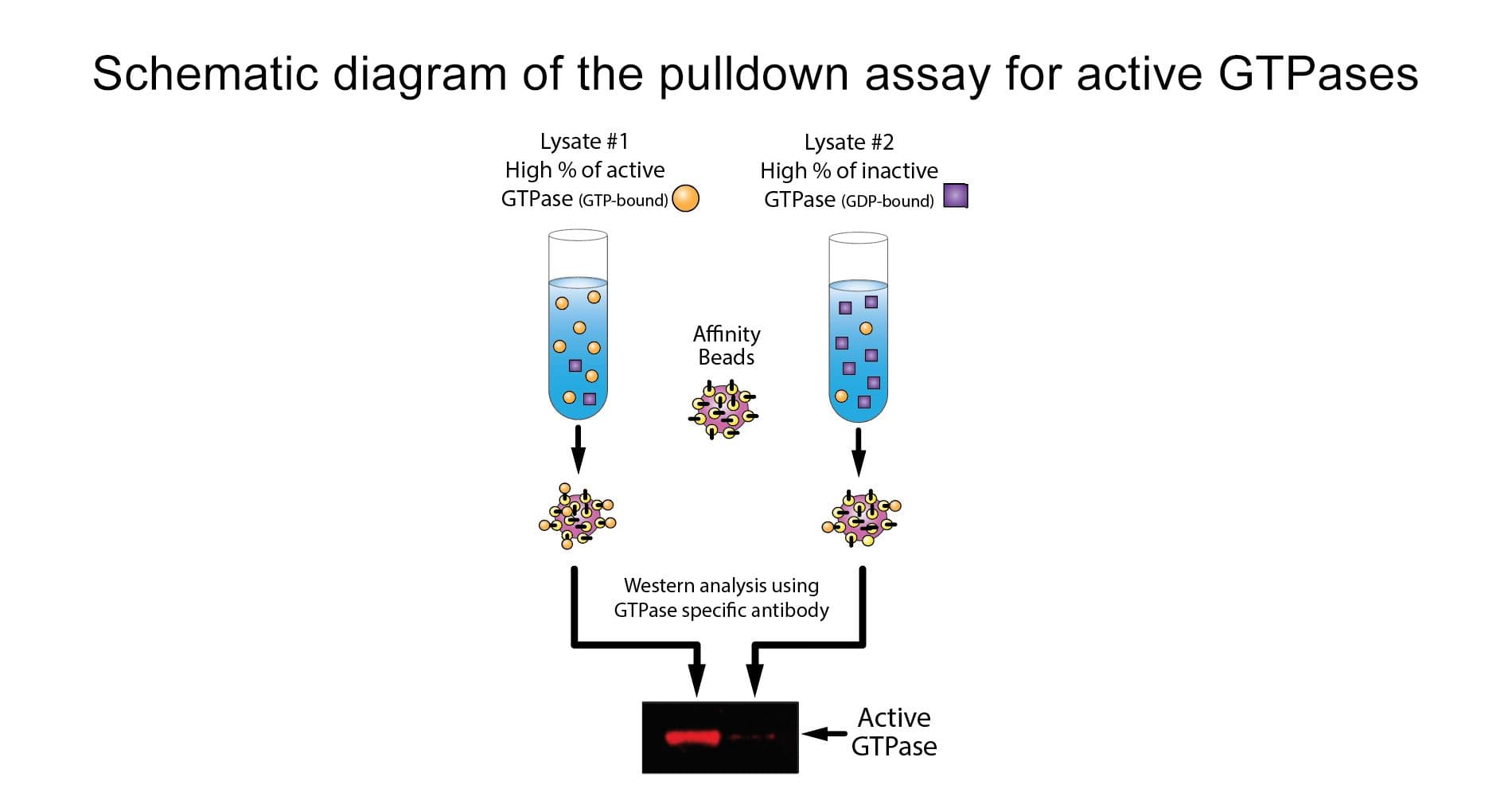
The Cdc42 pull-down assay isolates active Cdc42/Rac (GTP-bound form) using the Cdc42/Rac-binding domain (PBD) of an effector protein immobilized on agarose beads. The bound Cdc42 is then detected and quantified by Western blotting using a Cdc42-specific antibody.
Key characteristics
Cat. #BK034


© 2026 Cytoskeleton, Inc All Rights Reserved.