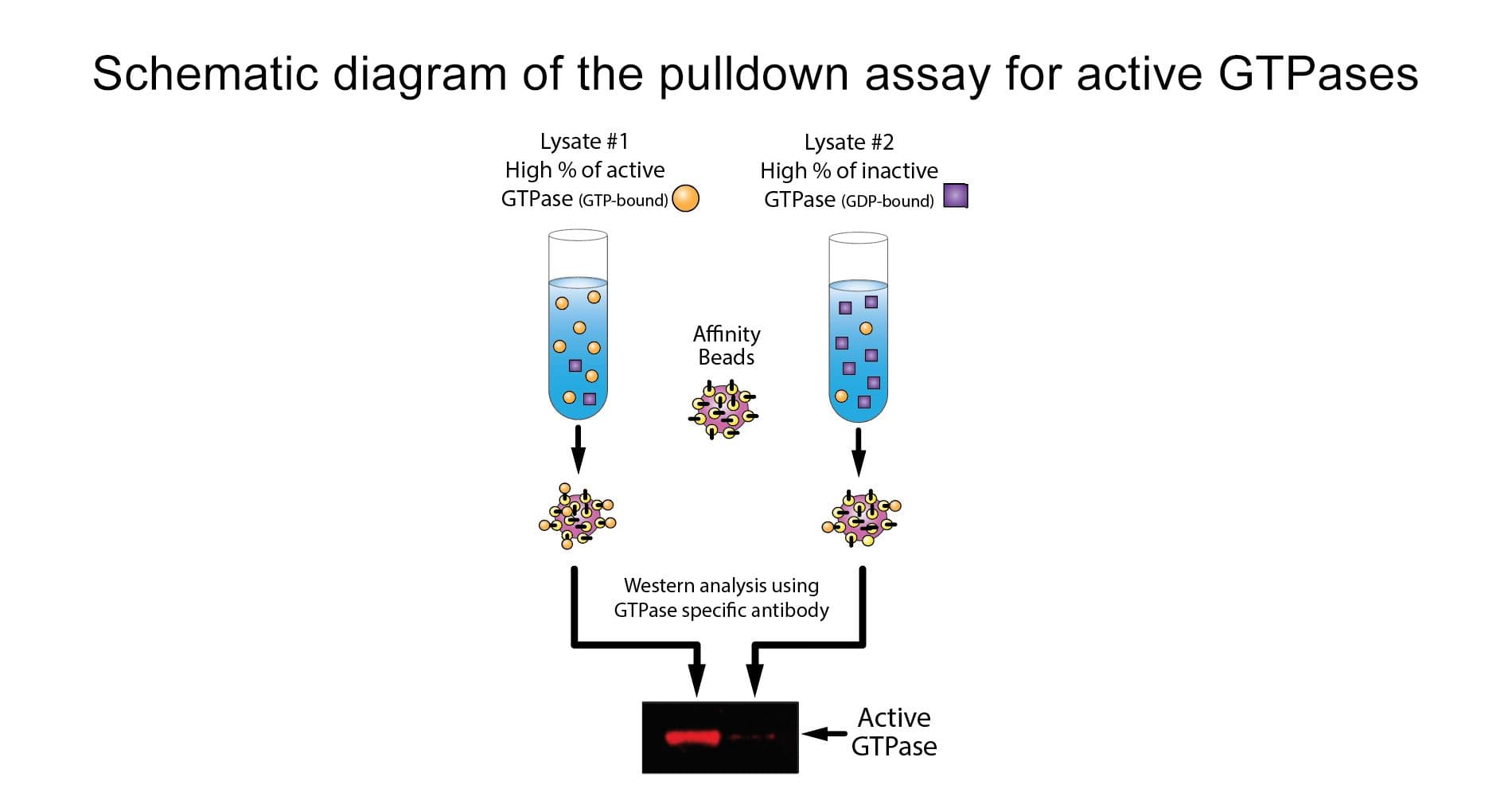
Kit contents (20-50 assays)
- Raf-RBD beads for affinity purification of active Ras
- Anti pan Ras antibody to detect activated Ras isotypes (H-, K-, and N). End user isotype specific antibody can be used for Ras specific isotype detection
- His-H-Ras control protein
- Cell lysis buffer-optimized for maintenance of GTP-bound GTPases
- Wash buffer-optimized for maintenance of GTP-bound GTPases
- Loading buffer: for control reactions
- STOP buffer: for control reactions
- GTPγS: for control reactions
- GDP: for control reactions
- Protease inhibitor cocktail
- DMSO: for protease resuspension
Equipment & materials required
- Cell lysates
- Cell scraper for harvesting cells
- Liquid nitrogen for snap freezing lysates
- Equipment for Western blot performance & analysis
- Microcentrifuge