Ras homolog gene family, member C (RhoC) is one of the three isoforms of the Rho family of GTPases, which is a sub-family of the larger Ras super-family of GTPases. The RhoC gene contains 6 exons and 5 introns. Three alternative transcripts encoding the same protein have been identified for the RhoC gene. The primary protein sequences of Rho-subfamily GTPases (RhoA, B and C) are about 85% identical, with most of the divergence within the C-termini. The sequence divergence among the three Rho isoforms is found in the insert loop, a helix between amino acids 123 and 137. The RhoC GTPase consists of 193 amino acids corresponding to a molecular weight of approximately 22 kDa. RhoC consists of a GTPase binding domain in the N-terminus. The C-terminus consists of geranylgeranyl group and carboxyl methylation extension, contains the sequence motif of GTP-binding proteins, binds to GDP and GTP with high affinity, and is involved in cycling between in-active, GDP-bound and active, GTP-bound states. RhoC protein is targeted by the Clostridium botulinum toxin exoenzyme C3 transferase, which modifies amino acid Asn41, rendering the RhoC protein irreversibly inactive. RhoC is mainly localized to the cytoplasm, though there is a small fraction localized to the plasma membrane of the Rat-2 fibroblast cells and associated with undefined perinuclear structures.
Like the RhoA and RhoB isoforms, RhoC is involved in multiple cellular processes, such as organization of the cytoskeletal components, cell division, or intracellular trafficking. A role for RhoC has also been observed in limb development. The RhoC protein has been implicated in cancer development. It is up-regulated in malignant pancreatic ductal carcinoma, inflammatory breast cancer tumors, and highly metastatic melanoma. Ectopic over-expression of RhoC increases the tumorigenic and metastasis properties of tumor progenitor cells. RhoC also induces the expression of angiogenic factors in human mammary epithelial cells by facilitating the vascularization of tumors in which it is expressed. RhoC protein is over-expressed in bladder carcinoma, breast carcinoma, and the squamous cell carcinoma of the head and neck. RhoC mRNA is over-expressed in adenocarcinoma of the ovary, pancreatic cancer, and hepatocellular carcinoma.
RhoC Images
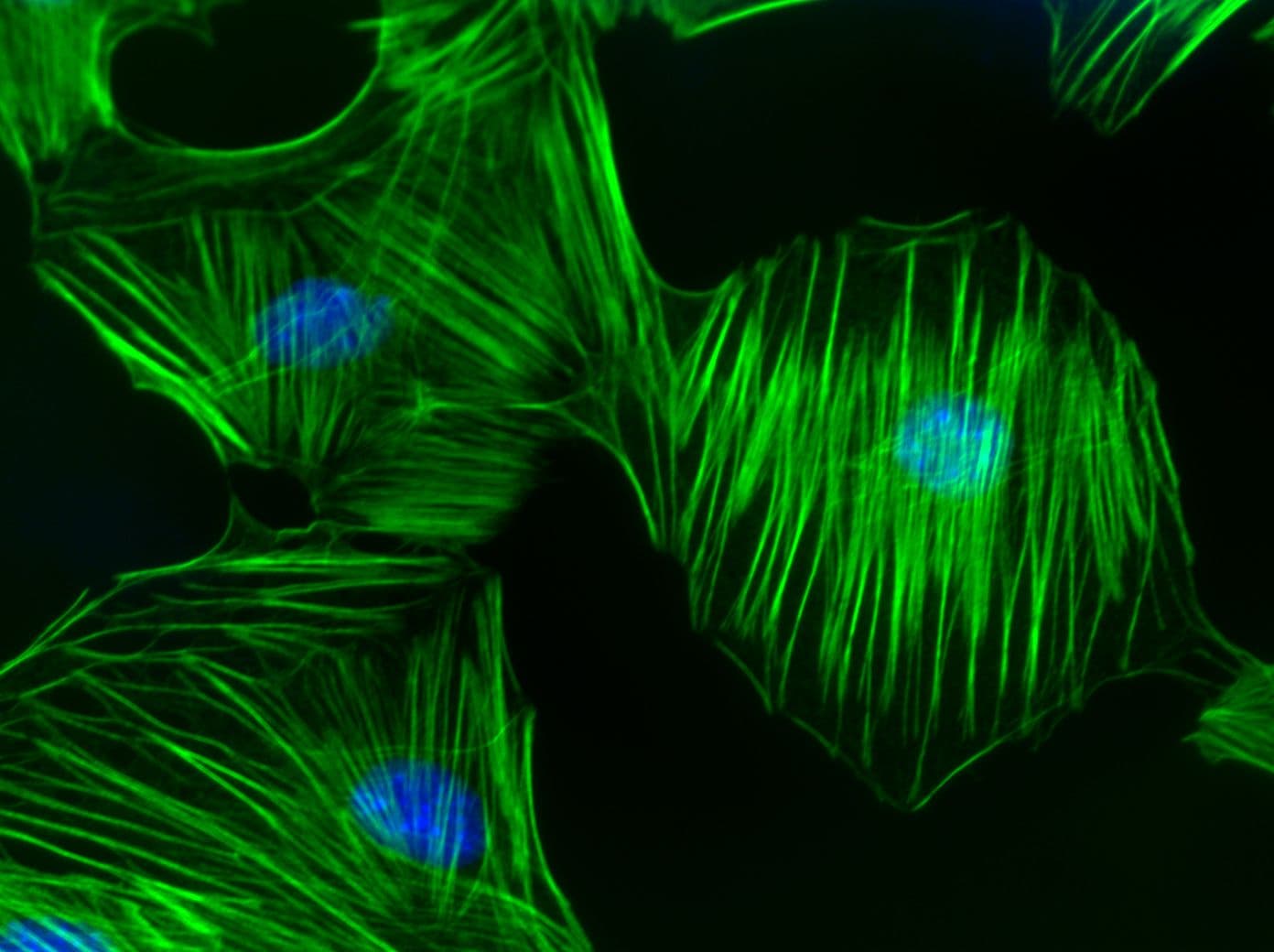
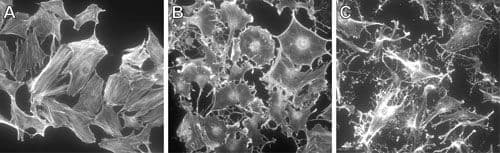
For more information on RhoC, please see here:
Benitah S.A. et al. 2004. Rho GTPases in human cancer: an unresolved link to upstream and downstream transcriptional regulation. Biochim. Biophys. Acta. 1705, 121-132.
Bishop A.L. and Hall A. 2000. Rho GTPases and their effector proteins. Biochem. J. 348, 241-255.
Bos J.L. et al. 2007. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 129, 865-877.
Bustelo X.R. et al. 2007. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 29, 356-370.
Chi X. et al. 2013. Roles of rho GTPases in intracellular transport and cellular transformation. Int. J. Mol. Sci. 14, 7089-7108.
Etienne-Manneville S. and Hall A. 2002. Rho GTPases in cell biology. Nature. 420, 629-635.
Fortin Ensign S.P. et al. 2013. Implications of Rho GTPase signaling in glioma Cell invasion and tumor progression. Front. Oncol. 3, 241.
Fritz G. et al. 2002. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br. J. Cancer. 87, 635-644.
Fritz G. et al. 1999. Rho GTPases are over-expressed in human tumors. Int. J. Cancer. 81, 682-687.
Gomez del Pulgar T. et al. 2005. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays. 27, 602-613.
Machacek M. et al. 2009. Coordination of Rho GTPase activities during cell protrusion. Nature. 461, 99-103.
Ridley A.J. et al. 2003. Cell migration: integrating signals from front to back. Science. 302, 1704-1709.
Rossman K.L. et al. 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167-180.
Wennerberg K. and Der C.J. 2004. Rho-family GTPases: it's not only Rac and Rho (and I like it). J. Cell Sci. 117, 1301-1312.
RhoC Related Products
Rho Activator I (Cat. # CN01)
Rho Activator II (Cat. # CN03)
Rho/Rac/Cdc42 Activator I(Cat. # CN04)
Acti-stain 488 phalloidin (Cat. # PHDG1-A)
Acti-stain 555 phalloidin (Cat. # PHDH1-A)
Acti-stain 670 phalloidin (Cat. # PHDN1-A)
Rhodamine Phalloidin (Cat. # PHDR1)
Anti-RhoA: mouse IgM Mab (Cat. # ARH05)
SiR-Actin Kit 50 nmol SiR-Actin and 1 umol verapamil (Cat. # CY-SC001)
RhoA Pull-down Activation Assay Biochem Kit (bead pull-down format) - 80 Assays (Cat. # BK036)
RhoA Pull-down Activation Assay Biochem Kit (bead pull-down format) - 80 Assays (Cat. # BK036-S)
RhoA G-LISA Activation Assay (Luminescence format) 96 assays (Cat. # BK121)
RhoA G-LISA Activation Assay Kit (Colorimetric format) 96 assays (Cat. # BK124)
RhoA / Rac1 / Cdc42 G-LISA Activation Assay Bundle 3 Kits (24 assays per kit) (Cat. # BK135)