Cdc42, A Rho GTPase and Regulator of Actin-Based Cytoskeletal Dynamics
Cdc42 is a 21.3 kDa, small GTPase protein encoded by 191 amino acids that belongs to the Rho sub-family of Ras super-family GTPases. There are two isoforms produced by alternative splicing with isoform 1 being the most commonly studied and expressed ubiquitously. Conversely, isoform 2 is restricted to the brain (also known as brain isoform, Cdc42b or G25K), but has been chosen as the 'canonical' sequence. Cdc42 switches between an active GTP-bound state and inactive GDP-bound state. This cycle is regulated by its intrinsic GTPase activity and its interaction with guanine exchange factors (GEFs) which promote the exchange of GDP for GTP and GTPase activating proteins (GAPs) which activate Cdc42’s GTP hydrolysis activity. Cdc42 localizes to membranes via a post-translational C-terminal geranylgeranyl lipid modification; however, it can be found as a soluble complex association with RhoGDI (Rho GDP-dissociation inhibitor), another means of regulating Cdc42’s activation. RhoGDIs sequester Cdc42-GDP in the cytoplasm through a transfer of geranylgeranyl moiety from membrane to GDI and inhibit its spontaneous GDP/GTP exchange activity. Additionally, Cdc42 can be phosphorylated by diverse kinases which regulate its interaction with RhoGDIs. To date over 70 Rho GEFs, 60 Rho GAPs and 3 RhoGDIs have been identified in mammals, reflecting the complexity of regulation of these classes of proteins.
Activation of Cdc42 is part of a signaling cascade initiated by stimulation of cell surface receptors and cell adhesion molecules which include tyrosine kinase receptors, heterodimeric G-protein-coupled receptors (GPCRs), cytokine receptors, integrins, and physical and chemical stresses. After GEF-mediated nucleotide exchange, GTP-bound Cdc42 can interact with a variety of downstream effector proteins, of which at least 23 have been identified. Putative effectors for Cdc42 include: p70 S6 kinase, MLK2 and MLK3, MEKK1 and MEKK4, PAK1, PAK2, PAK3 and PAK4, MRCKα and MRCKβ, ACK1 and ACK2, PI3K, PLD, PLC-β2, WASP and N-WASP, MSE55 and BORGs, IQGAP1 and IQGAP2, and CIP-4.
Cdc42 plays a significant role in a wide variety of cellular processes that are dependent on the actin cytoskeleton, such as cytokinesis, phagocytosis, cell migration, morphogenesis, chemotaxis, axon guidance, axon myelination, intracellular trafficking, gene transcription, cell-cycle regulation and cell fate determination. In regards to cell motility, Cdc42 is directly involved in the formation of filopodia via interactions with actin binding proteins which are nucleation factors). However, the contribution of Cdc42 in these different cellular processes could be cell-type specific. Dysregulation of Cdc42 is found in several pathogenic processes such as cancer, neurodegenerative disorders, and cardiovascular disease.
Cdc42’s role in cancer is dependent on over-expression and/or over-activation rather than activating mutations. Cdc42 contributes to cancer development through defects or dysfunction in its physiological roles. For instance, alterations in Cdc42-mediated intracellular trafficking - especially through the regulation of the processing and degradation of EGF receptor, cell cycle regulation and survival, polarity, migration, and transcriptional control is related to oncogenesis. The contribution of Cdc42 to cancer progression seems to be tissue-specific since Cdc42 appears to have pro-oncogenic and anti-oncogenic properties depending on cellular context. However, there are more cell types where Cdc42 is a pro-oncogenic factor. Cdc42 protein levels are significantly elevated in breast cancer tissue vs normal tissue, testicular tumor tissue, head and neck squamous cell carcinomas, melanomas, colorectal cancer, and hepatocellular carcinoma.
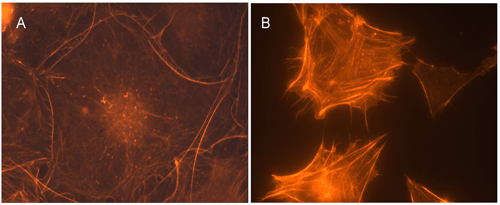
Swiss 3T3 fibroblasts plated on coverslips at 1000 cells / cm2 and grown for two days in DMEM plus 10% fetal calf serum at 37°C and 5% CO2, were serum starved for 16 h in media containing 1% serum and 8 h in 0% serum media. Cultures were treated with 5 µl of CN02 per ml of medium for 10 min at 37°C. Cells were then fixed, stained with rhodamine-labeled phalloidin (Cat. # PHDR1 or BK005), and visualized by fluorescence microscopy. Images were taken at a magnification of 40×. The untreated control cells were treated with 5ul sterile PBS per ml of medium. The cells treated with CN02 produced abundant ruffles and lamellipodia whereas the control had less than 10% of CN02 levels of similar actin structures. Under similar conditions the activity of Cdc42 and Rac increased by 50 and 130% respectively as measured by the G-LISATM Activation Assays (Cat.# BK127 and BK125 resp.). A = serum starved cell, 2 s exposure; B = example Rac activation, 0.3 s exposure.
For more information about Cdc42, please see here:
Bishop A.L. and Hall A. 2000. Rho GTPases and their effector proteins. Biochem. J. 348, 241-255.
Cerione R.A. 2004. Cdc42: new roads to travel. Trends Cell Biol. 14, 127-132.
Fritz G. et al. 1999. Rho GTPases are over-expressed in human tumors. Int. J. Cancer. 81, 682-687.
Gupton S.L. and Gertler F.B. 2007. Filopodia: the fingers that do the walking. Sci. STKE. 2007, re5.
Vega F.M. and Ridley A.J. 2008. Rho GTPases in cancer cell biology. FEBS Lett. 582, 2093-2101
Related Products
GLISAs and Pull-Downs
Cdc42 Pull-down Activation Assay Biochem Kit (bead pull-down format) (Cat. # BK034)
Cdc42 G-LISA Activation Assay (Colorimetric format) (Cat. # BK127)
RhoA / Rac1 / Cdc42 Activation Assay Combo Biochem Kit (bead pull-down format) (Cat. # BK030)
RhoA / Rac1 / Cdc42 G-LISA Activation Assay Bundle 3 Kits (Cat. #BK135)
Small G-Proteins
Cdc42 protein: GST tagged: human dominant negative (Cat. #C17G01)
Cdc42 protein: His tagged: human constitutively active (Cat. #C6101)
Cdc42 protein: His tagged: human wild type (Cat. #CD01)
Cdc42 protein: GSt tagged: human wid type (Cat. #CDG01)
Antibodies and Beads
Anti-Cdc42: mouse Mab (Cat. # ACD03)
PAK-PBD protein, GST-tagged (Cat. #PAK01)
PAK-PBD beads (binds active Rac/Cdc42 proteins) (Cat. #PAK02)
G-Switch Activators and Inhibitors
Rac/Cdc42 Activator II (Cat. #CN02)
Rho/Rac/Cdc42 Activator I (Cat. #CN04)
Live Cell Imaging Probes
Spirochrome SiR-Actin Kit (Cat. #CY-SC001)
Phalloidins
Acti-stain 488 Phalloidin (Cat. #PHDG1)
Acti-stain 555 Phalloidin (Cat. #PHDH1)
