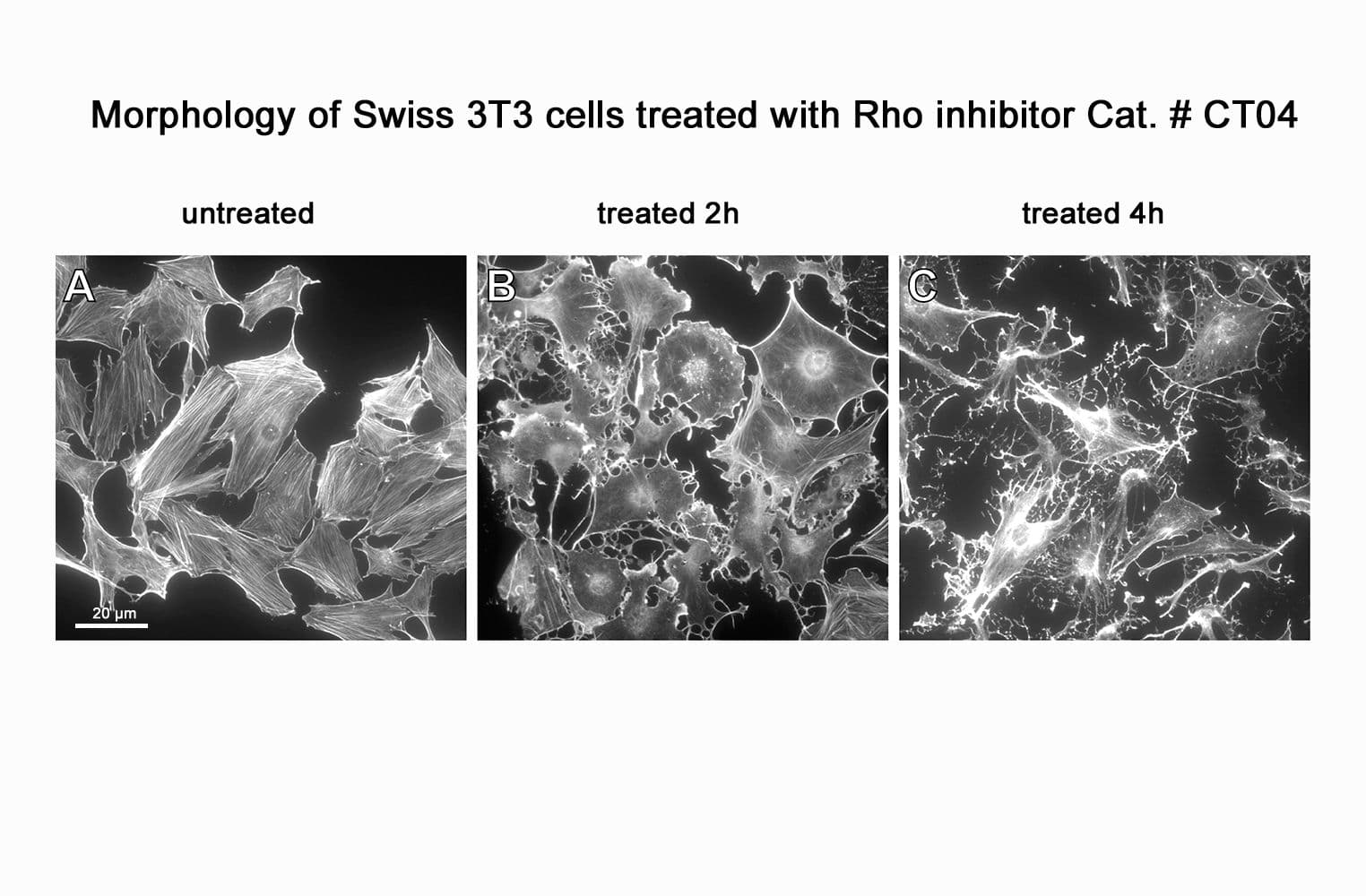
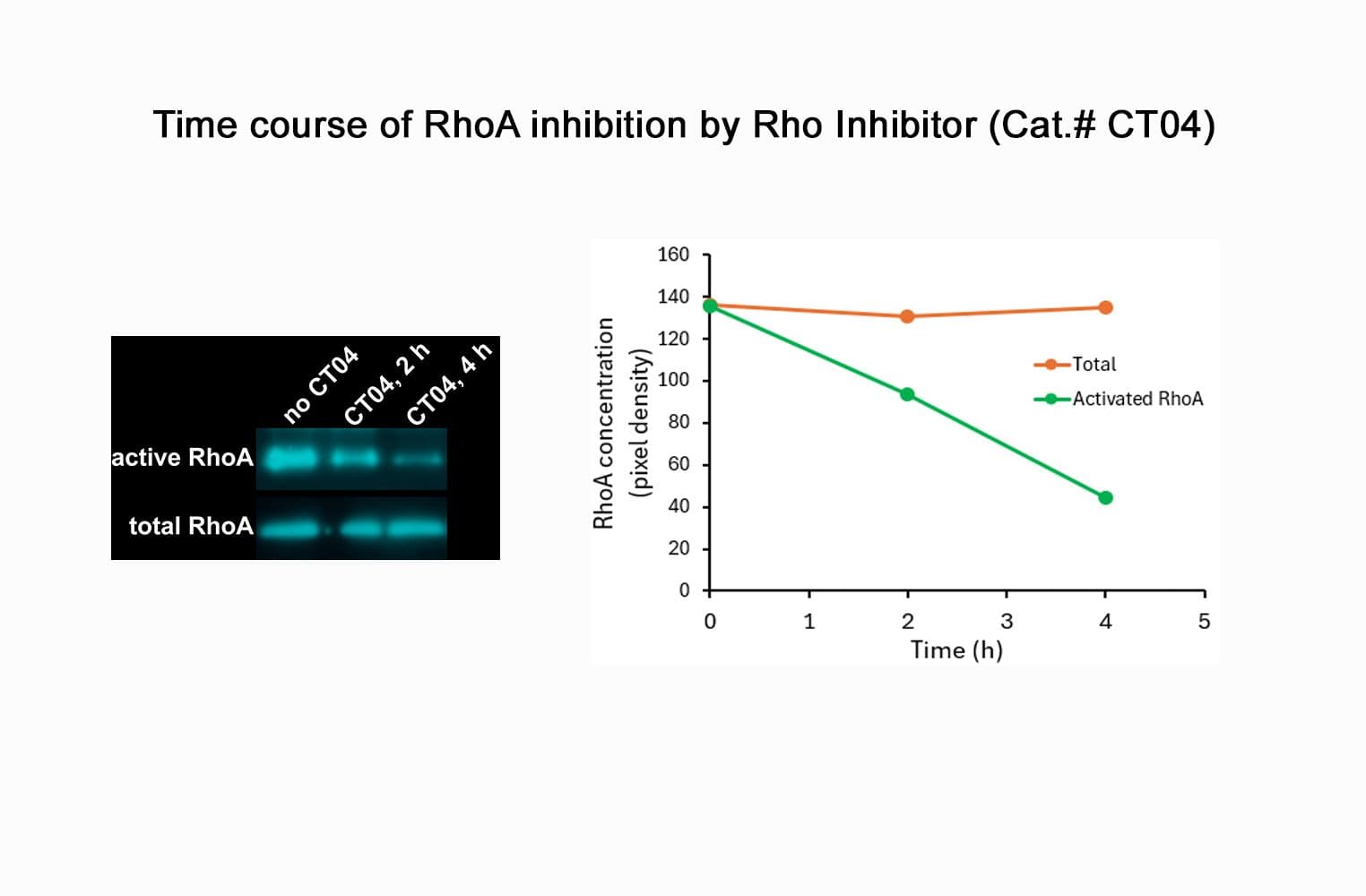
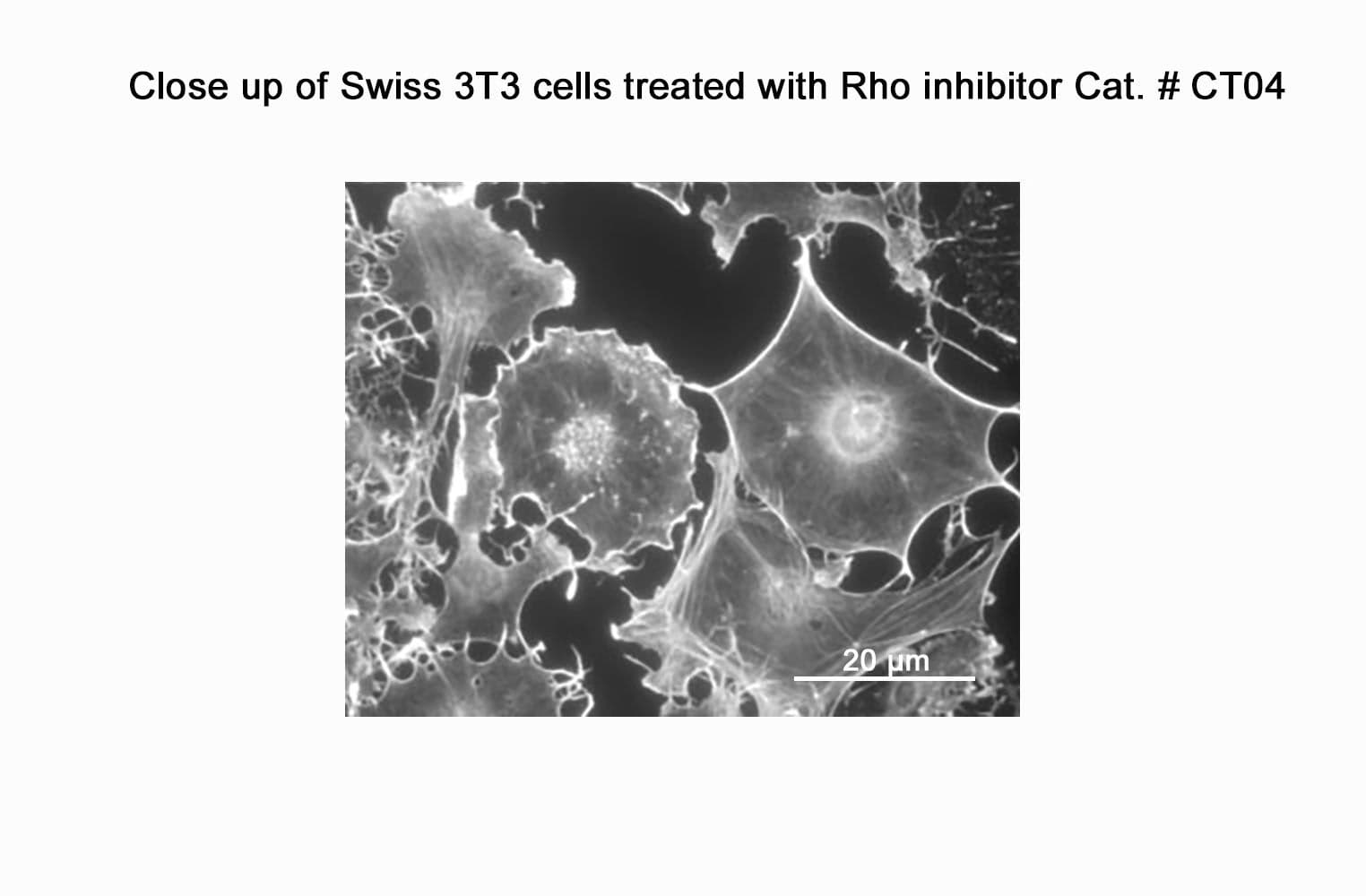
CT04, part of the G-Switch™ line, is a modified C3 Transferase from Clostridium botulinum covalently linked to a proprietary cell penetrating moiety. It specifically inhibits RhoA, B and C by ADP-ribosylating asparagine 41 in the GTPase effector-binding domain. It will not inhibit other Rho family proteins such as Rac or Cdc42. Unlike native exoenzyme, which requires ~24h to inactivate Rho, CT04 can inactivate 75-95% within 2-4 hours.
Produced in a bacterial expression system, CT04 is a 24 kDa recombinant protein with an N-terminal six-histidine tag.
Preparation & Use:
Protein purity is assessed by scanning densitometry of Coomassie Blue stained protein on a 4-20% polyacrylamide gel. Purity was determined to be >90%.
Biological activity of CT04 is demonstrated by its ability to robustly inactivate RhoA in live Swiss 3T3 cells. Treatment at 2 µg/ml for 4h produces >80% inhibition of RhoA activation by calpeptin as determined by a RhoA G-LISA assay BK124.
CT04 has been shown to inactivate Rho to an efficiency of 75-95% in fibroblasts, neurons, epithelial, endothelial, and hematopoietic cells as well as other primary and immortalized cell lines.
Responses to CT04 will vary depending on the cell line used.
Cat. #CT04